Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2017-07-25 , DOI: 10.1016/j.apcatb.2017.07.073 Lei Wang , Haiqin Yue , Zelin Hua , Haoyi Wang , Xiaobao Li , Licheng Li
|
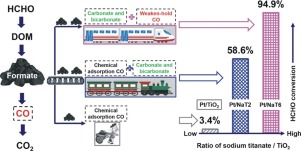
A series of titania nanowires containing different Na content (NaxTiO2) were prepared from sodium titanate by varying the pH value of ion-exchange. They were used to load the Pt nanoparticles for low temperature formaldehyde catalytic oxidation. As prepared samples were systematically characterized by X-ray diffraction (XRD), transmission electron microscope (TEM), X-ray photoelectron spectroscopy (XPS), in situ diffuse-reflectance infrared Fourier transform spectroscopy (DRIFTS), temperature programmed reduction/desorption/oxidation (TPR/TPD/TPO) and so on. The results showed that NaxTiO2 were consisted of anatase and sodium titanate. As pH value increased, the surface area and anatase content of catalyst decreased. Pt particles on NaxTiO2 were more easily oxidized and had smaller size but less exposed atoms compared with those on pure TiO2. The HCHO catalytic oxidation result exhibited that Pt/NaxTiO2 catalysts possessed excellent HCHO catalytic oxidation at ambient temperature that were 8.9–52.8 times the activities as that of Pt/TiO2. Kinetic studies manifested the easy activation of HCHO reaction over Pt/NaxTiO2 catalysts. Structure-performance analysis showed that the enhanced performance of Pt/NaxTiO2 catalysts was mainly attributed to two factors: increase in HCHO adsorption amount and change in HCHO decomposition pathway. According to HCHO-DRIFTS, linearly adsorbed CO generated from formate can react with hydroxyls to be carbonate/bicarbonate over Pt/NaxTiO2 catalysts. Simultaneously, the formate in Pt/NaxTiO2 catalyst with high Na content was also decomposed into a weak adsorbed CO. Both reaction pathways avoided the strong adsorption of CO on Pt/NaxTiO2 catalysts, which can facilitate the HCHO decomposition.
中文翻译:

用于低温甲醛分解的高活性Pt / Na x TiO 2催化剂
通过改变离子交换的pH值,由钛酸钠制备了一系列含有不同Na含量的二氧化钛纳米线(Na x TiO 2)。它们被用于负载Pt纳米颗粒,用于低温甲醛催化氧化。通过X射线衍射(XRD),透射电子显微镜(TEM),X射线光电子能谱(XPS),原位漫反射红外傅里叶变换光谱(DRIFTS),程序升温还原/解吸/氧化(TPR / TPD / TPO)等。结果表明,Na x TiO 2由锐钛矿和钛酸钠组成。随着pH值的增加,催化剂的表面积和锐钛矿含量降低。与纯TiO 2相比,Na x TiO 2上的Pt颗粒更容易被氧化,尺寸更小,但暴露的原子更少。HCHO催化氧化结果表明,Pt / Na x TiO 2催化剂在环境温度下具有优异的HCHO催化氧化能力,是Pt / TiO 2活性的8.9-52.8倍。动力学研究表明,在Pt / Na x TiO 2催化剂上HCHO反应易于活化。结构性能分析表明,Pt / Na的性能增强x TiO 2催化剂主要归因于两个因素:HCHO吸附量的增加和HCHO分解途径的改变。根据HCHO-DRIFTS,在Pt / Na x TiO 2催化剂上,由甲酸盐生成的线性吸附的CO可以与羟基反应生成碳酸盐/碳酸氢盐。同时,高Na含量的Pt / Na x TiO 2催化剂中的甲酸酯也被分解为弱吸附的CO。两种反应路径均避免了CO在Pt / Na x TiO 2催化剂上的强吸附,从而促进了HCHO的分解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号