当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Light‐Triggered Clustered Vesicles with Self‐Supplied Oxygen and Tissue Penetrability for Photodynamic Therapy against Hypoxic Tumor
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2017-07-24 , DOI: 10.1002/adfm.201702108 Junjie Li 1 , Kai Wei 1 , Shuai Zuo 1 , Yixuan Xu 1 , Zengshi Zha 1 , Wendong Ke 1 , Huabing Chen 2 , Zhishen Ge 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2017-07-24 , DOI: 10.1002/adfm.201702108 Junjie Li 1 , Kai Wei 1 , Shuai Zuo 1 , Yixuan Xu 1 , Zengshi Zha 1 , Wendong Ke 1 , Huabing Chen 2 , Zhishen Ge 1
Affiliation
|
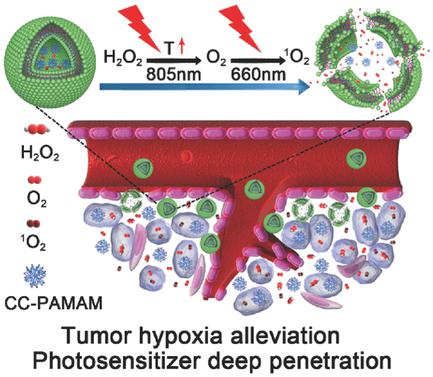
Smart nanocarriers are of particular interest for highly effective photodynamic therapy (PDT) in the field of precision nanomedicine. Nevertheless, a critical challenge still remains in the exploration of potent PDT treatment against hypoxic tumor. Herein, light‐triggered clustered polymeric vesicles for photoinduced hypoxic tumor ablation are demonstrated, which are able to deeply penetrate into the tumor and simultaneously afford oxygen supply upon light irradiation. Hydrogen peroxide (H2O2) and poly(amidoamine) dendrimer conjugating chlorin e6/cypate (CC‐PAMAM) are coassembled with reactive‐oxygen‐species‐responsive triblock copolymer into the polymeric vesicles. Upon 805 nm irradiation, the vesicles exhibit the light‐triggered thermal effect that is able to decompose H2O2 into O2, which distinctly ensures the alleviation of tumor hypoxia at tumor. Followed by 660 nm irradiation, the vesicles are rapidly destabilized through singlet oxygen‐mediated cleavage of copolymer under light irradiation and thus allow the release of photoactive CC‐PAMAM from the vesicular chambers, followed by their deep penetration in the poorly permeable tumor. Consequently, the light‐triggered vesicles with both self‐supplied oxygen and deep tissue penetrability achieve the total ablation of hypoxic hypopermeable pancreatic tumor through photodynamic damage. These findings represent a general and smart nanoplatform for effective photoinduced treatment against hypoxic tumor.
中文翻译:

轻触发的簇状囊泡,具有自供氧和组织穿透性,可用于对缺氧肿瘤进行光动力治疗。
智能纳米载体对于精密纳米医学领域中的高效光动力疗法(PDT)尤为重要。然而,在探索针对缺氧肿瘤的有效PDT治疗方面仍然存在着严峻的挑战。在本文中,展示了用于光诱导的缺氧肿瘤消融的光触发簇状聚合物囊泡,它们能够深入地渗透到肿瘤中,并在光照射时同时提供氧气。过氧化氢(H 2 O 2)和聚(酰胺基胺)树枝状大分子结合二氢卟酚e6 /环戊二酸酯(CC-PAMAM)与反应性氧物种反应性三嵌段共聚物共组装到聚合物囊泡中。在805 nm照射下,囊泡表现出光触发的热效应,能够分解H将2 O 2转换为O 2,可明显确保减轻肿瘤处的缺氧。在660 nm辐照之后,在光辐照下,通过单线态氧介导的共聚物裂解,囊泡迅速失稳,从而使光敏CC-PAMAM从囊泡腔中释放出来,然后在渗透性差的肿瘤中深度渗透。因此,具有自供氧和深层组织穿透性的光触发囊泡通过光动力损伤实现了对缺氧性低渗透性胰腺肿瘤的完全消融。这些发现代表了针对缺氧肿瘤的有效光诱导治疗的通用且智能的纳米平台。
更新日期:2017-07-24
中文翻译:

轻触发的簇状囊泡,具有自供氧和组织穿透性,可用于对缺氧肿瘤进行光动力治疗。
智能纳米载体对于精密纳米医学领域中的高效光动力疗法(PDT)尤为重要。然而,在探索针对缺氧肿瘤的有效PDT治疗方面仍然存在着严峻的挑战。在本文中,展示了用于光诱导的缺氧肿瘤消融的光触发簇状聚合物囊泡,它们能够深入地渗透到肿瘤中,并在光照射时同时提供氧气。过氧化氢(H 2 O 2)和聚(酰胺基胺)树枝状大分子结合二氢卟酚e6 /环戊二酸酯(CC-PAMAM)与反应性氧物种反应性三嵌段共聚物共组装到聚合物囊泡中。在805 nm照射下,囊泡表现出光触发的热效应,能够分解H将2 O 2转换为O 2,可明显确保减轻肿瘤处的缺氧。在660 nm辐照之后,在光辐照下,通过单线态氧介导的共聚物裂解,囊泡迅速失稳,从而使光敏CC-PAMAM从囊泡腔中释放出来,然后在渗透性差的肿瘤中深度渗透。因此,具有自供氧和深层组织穿透性的光触发囊泡通过光动力损伤实现了对缺氧性低渗透性胰腺肿瘤的完全消融。这些发现代表了针对缺氧肿瘤的有效光诱导治疗的通用且智能的纳米平台。






























 京公网安备 11010802027423号
京公网安备 11010802027423号