当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2D–3D structural transition in sub-nanometer PtN clusters supported on CeO2(111)
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-06-13 00:00:00 , DOI: 10.1039/c7cp02753b Lauro Oliver Paz-Borbón 1, 2, 3, 4 , Andres López-Martínez 1, 2, 3, 4 , Ignacio L. Garzón 1, 2, 3, 4 , Alvaro Posada-Amarillas 4, 5, 6, 7 , Henrik Grönbeck 8, 9, 10, 11
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-06-13 00:00:00 , DOI: 10.1039/c7cp02753b Lauro Oliver Paz-Borbón 1, 2, 3, 4 , Andres López-Martínez 1, 2, 3, 4 , Ignacio L. Garzón 1, 2, 3, 4 , Alvaro Posada-Amarillas 4, 5, 6, 7 , Henrik Grönbeck 8, 9, 10, 11
Affiliation
|
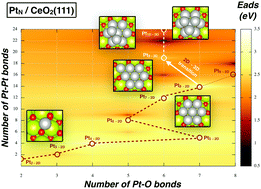
Transition metal particles dispersed on oxide supports are used as heterogeneous catalysts in numerous applications. One example is platinum clusters supported on ceria which is used in automotive catalysis. Although control at the nm-scale is desirable to open new technological possibilities, there is limited knowledge both experimentally and theoretically regarding the geometrical structure and stability of sub-nanometer platinum clusters supported on ceria. Here we report a systematic, Density Functional Theory (DFT) study on the growth trends of CeO2(111) supported PtN clusters (N = 1–10). Using a global optimization methodology as a guidance tool to locate putative global minima, our results show a clear preference for 2D planar structures up to size Pt8. It is followed by a structural transition to 3D configurations at larger sizes. This remarkable trend is explained by the subtle competition between the formation of strong Pt–O bonds and the cluster internal Pt–Pt bonds. Our calculations show that the reducibility of CeO2(111) provides a mechanism to anchor PtN clusters where they become oxidized in a two-way charge transfer mechanism: (a) an oxidation process, where Osurface atoms withdraw charge from Pt atoms forming Pt–O bonds, (b) surface Ce4+ atoms are reduced, leading to Ce3+. The active role of the CeO2(111) support in modifying the structural and eventually the chemical properties of sub-nanometer PtN clusters is computationally demonstrated.
中文翻译:

CeO 2支持的亚纳米Pt N团簇中的2D–3D结构转变(111)
分散在氧化物载体上的过渡金属颗粒在许多应用中用作多相催化剂。一个例子是负载在二氧化铈上的铂簇,用于汽车催化。尽管需要在纳米级进行控制以开辟新的技术可能性,但是在实验和理论上,关于负载在二氧化铈上的亚纳米铂簇的几何结构和稳定性的知识有限。在这里,我们报告了有关CeO 2(111)支撑的Pt N团簇(N = 1-10)的生长趋势的系统的密度泛函理论(DFT)研究。使用全局优化方法作为指导工具来定位推定的全局最小值,我们的结果表明,明显首选尺寸最大为Pt的2D平面结构8。随后是从结构上过渡到更大尺寸的3D配置。强大的Pt-O键的形成与簇内部的Pt-Pt键之间的微妙竞争可以解释这种非凡的趋势。我们的计算表明,CeO 2(111)的可还原性提供了一种机制,可将Pt N团簇锚固在一种双向电荷转移机制中被氧化的位置:(a)氧化过程,其中O表面原子从形成的Pt原子中撤出电荷Pt–O键,(b)表面Ce 4+原子被还原,导致Ce 3+。CeO 2(111)载体在修饰亚纳米Pt的结构以及最终化学性质方面的积极作用通过计算证明了N个群集。
更新日期:2017-06-29
中文翻译:

CeO 2支持的亚纳米Pt N团簇中的2D–3D结构转变(111)
分散在氧化物载体上的过渡金属颗粒在许多应用中用作多相催化剂。一个例子是负载在二氧化铈上的铂簇,用于汽车催化。尽管需要在纳米级进行控制以开辟新的技术可能性,但是在实验和理论上,关于负载在二氧化铈上的亚纳米铂簇的几何结构和稳定性的知识有限。在这里,我们报告了有关CeO 2(111)支撑的Pt N团簇(N = 1-10)的生长趋势的系统的密度泛函理论(DFT)研究。使用全局优化方法作为指导工具来定位推定的全局最小值,我们的结果表明,明显首选尺寸最大为Pt的2D平面结构8。随后是从结构上过渡到更大尺寸的3D配置。强大的Pt-O键的形成与簇内部的Pt-Pt键之间的微妙竞争可以解释这种非凡的趋势。我们的计算表明,CeO 2(111)的可还原性提供了一种机制,可将Pt N团簇锚固在一种双向电荷转移机制中被氧化的位置:(a)氧化过程,其中O表面原子从形成的Pt原子中撤出电荷Pt–O键,(b)表面Ce 4+原子被还原,导致Ce 3+。CeO 2(111)载体在修饰亚纳米Pt的结构以及最终化学性质方面的积极作用通过计算证明了N个群集。











































 京公网安备 11010802027423号
京公网安备 11010802027423号