当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and formulation studies of griseofulvin analogues with improved solubility and metabolic stability
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-03-25 12:00:01 Asger B. Petersen, Nikolaj S. Andersen, Gleb Konotop, Nur Hafzan Md Hanafiah, Marc S. Raab, Alwin Krämer, Mads H. Clausen
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-03-25 12:00:01 Asger B. Petersen, Nikolaj S. Andersen, Gleb Konotop, Nur Hafzan Md Hanafiah, Marc S. Raab, Alwin Krämer, Mads H. Clausen
|
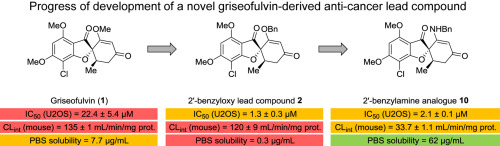
Griseofulvin (1) is an important antifungal agent that has recently received attention due to its antiproliferative activity in mammalian cancer cells. Comprehensive SAR studies have led to the identification of 2′-benzyloxy griseofulvin 2, a more potent analogue with low micromolar anticancer potency in vitro. Analogue 2 was also shown to retard tumor growth through inhibition of centrosomal clustering in murine xenograft models of colon cancer and multiple myeloma. However, similar to griseofulvin, compound 2 exhibited poor metabolic stability and aqueous solubility. In order to improve the poor pharmacokinetic properties, 11 griseofulvin analogues were synthesized and evaluated for biological activity and physiological stabilities including SGF, plasma, and metabolic stability. Finally, the most promising compounds were investigated in respect to thermodynamic solubility and formulation studies. The 2′-benzylamine analogue 10 proved to be the most promising compound with low μM in vitro anticancer potency, a 200-fold increase in PBS solubility over compound 2, and with improved metabolic stability. Furthermore, this analogue proved compatible with formulations suitable for both oral and intravenous administration. Finally, 2′-benzylamine analogue 10 was confirmed to induce G2/M cell cycle arrest in vitro.
中文翻译:

具有改善的溶解度和代谢稳定性的灰黄霉素的类似物的合成和制剂研究
灰黄霉素(1)是一种重要的抗真菌剂,由于其在哺乳动物癌细胞中的抗增殖活性,最近受到关注。全面的SAR研究已导致鉴定2'-苄氧基灰黄霉素2,这是一种更有效的类似物,在体外具有较低的微摩尔抗癌效力。在结肠癌和多发性骨髓瘤的小鼠异种移植模型中,类似物2还显示出通过抑制中心体簇而延迟了肿瘤的生长。然而,类似于灰黄霉素,化合物2表现出较差的代谢稳定性和水溶性。为了改善不良的药代动力学特性,合成了11种灰黄霉素的类似物,并对其生物学活性和生理稳定性进行了评估,包括SGF,血浆和代谢稳定性。最后,在热力学溶解度和配方研究方面对最有前途的化合物进行了研究。2'-苄胺类似物10被证明是最有前途的化合物,其体外抗癌潜能低,PBS溶解度比化合物2高200倍,并且代谢稳定性更高。此外,已证明该类似物与适用于口服和静脉内给药的制剂相容。最后,证实了2'-苄胺类似物10在体外诱导G2 / M细胞周期停滞。该类似物被证明与适用于口服和静脉内给药的制剂相容。最后,证实了2'-苄胺类似物10在体外诱导G2 / M细胞周期停滞。该类似物被证明与适用于口服和静脉内给药的制剂相容。最后,证实了2'-苄胺类似物10在体外诱导G2 / M细胞周期停滞。
更新日期:2017-03-26
中文翻译:

具有改善的溶解度和代谢稳定性的灰黄霉素的类似物的合成和制剂研究
灰黄霉素(1)是一种重要的抗真菌剂,由于其在哺乳动物癌细胞中的抗增殖活性,最近受到关注。全面的SAR研究已导致鉴定2'-苄氧基灰黄霉素2,这是一种更有效的类似物,在体外具有较低的微摩尔抗癌效力。在结肠癌和多发性骨髓瘤的小鼠异种移植模型中,类似物2还显示出通过抑制中心体簇而延迟了肿瘤的生长。然而,类似于灰黄霉素,化合物2表现出较差的代谢稳定性和水溶性。为了改善不良的药代动力学特性,合成了11种灰黄霉素的类似物,并对其生物学活性和生理稳定性进行了评估,包括SGF,血浆和代谢稳定性。最后,在热力学溶解度和配方研究方面对最有前途的化合物进行了研究。2'-苄胺类似物10被证明是最有前途的化合物,其体外抗癌潜能低,PBS溶解度比化合物2高200倍,并且代谢稳定性更高。此外,已证明该类似物与适用于口服和静脉内给药的制剂相容。最后,证实了2'-苄胺类似物10在体外诱导G2 / M细胞周期停滞。该类似物被证明与适用于口服和静脉内给药的制剂相容。最后,证实了2'-苄胺类似物10在体外诱导G2 / M细胞周期停滞。该类似物被证明与适用于口服和静脉内给药的制剂相容。最后,证实了2'-苄胺类似物10在体外诱导G2 / M细胞周期停滞。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号