当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
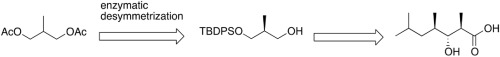
Preparation of (2R, 3R, 4R)-3-hydroxy-2,4,6-trimethylheptanoic acid via enzymatic desymmertization
Tetrahedron ( IF 2.1 ) Pub Date : 2016-12-08 08:28:11 Yoshinori Tokairin, Hiroyuki Konno
Tetrahedron ( IF 2.1 ) Pub Date : 2016-12-08 08:28:11 Yoshinori Tokairin, Hiroyuki Konno
|
Synthesis of a unique fatty acyl unit to build the N-terminus of callipeltin A and homophymine B is described. Our approach to access (2R, 3R, 4R)-3-hydroxy-2,4,6-trimethylheptanoic acid uses enzymatic hydrolysis for the desymmetrization of achiral acetate, followed by diastereoselective Roush crotylboration and Wittig olefination for the backbone construction.
中文翻译:

酶法脱甲基制备(2R,3R,4R)-3-羟基-2,4,6-三甲基庚酸
描述了独特的脂肪酰基单元的合成以构建卡培尔汀A和高草甘膦B的N-末端。我们获得(2R,3R,4R)-3-羟基-2,4,6-三甲基庚酸的方法是使用酶促水解进行非手性乙酸酯的去对称化,然后进行非对映选择性的Roush烷基硼酸酯化和Wittig烯烃化来构建骨架。
更新日期:2016-12-09
中文翻译:

酶法脱甲基制备(2R,3R,4R)-3-羟基-2,4,6-三甲基庚酸
描述了独特的脂肪酰基单元的合成以构建卡培尔汀A和高草甘膦B的N-末端。我们获得(2R,3R,4R)-3-羟基-2,4,6-三甲基庚酸的方法是使用酶促水解进行非手性乙酸酯的去对称化,然后进行非对映选择性的Roush烷基硼酸酯化和Wittig烯烃化来构建骨架。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号