当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium‐Ion‐Transfer Kinetics of Single LiMn2O4 Particles
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2016-12-06 , DOI: 10.1002/anie.201610485 Giorgia Zampardi 1 , Christopher Batchelor-McAuley 1 , Enno Kätelhön 1 , Richard G. Compton 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2016-12-06 , DOI: 10.1002/anie.201610485 Giorgia Zampardi 1 , Christopher Batchelor-McAuley 1 , Enno Kätelhön 1 , Richard G. Compton 1
Affiliation
|
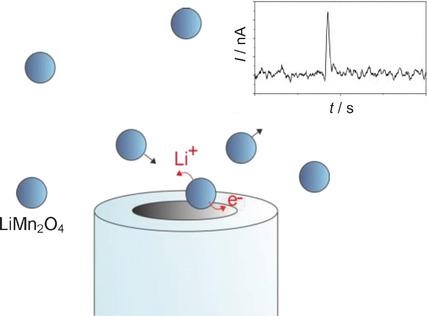
A stochastic investigation of lithium deinsertion from individual 200‐nm‐sized particles of LiMn2O4 reveals the rate‐determining step at high overpotentials to be the transfer of the cation across the particle–electrolyte interface. Measurement of the (electro)chemical behavior of the spinel is undertaken without forming a conductive composite electrode. The kinetics of the interfacial ion transfer defines a theoretical upper limit for the discharge rates of batteries using LiMn2O4 in an aqueous environment.
中文翻译:

LiMn2O4颗粒的锂离子转移动力学
对LiMn 2 O 4的单个200 nm大小的颗粒中的锂脱嵌的随机研究表明,在高超电势下,决定速率的步骤是阳离子在颗粒-电解质界面上的转移。在不形成导电复合电极的情况下进行尖晶石的(电)化学行为的测量。界面离子转移的动力学定义了在水环境中使用LiMn 2 O 4的电池放电速率的理论上限。
更新日期:2016-12-06
中文翻译:

LiMn2O4颗粒的锂离子转移动力学
对LiMn 2 O 4的单个200 nm大小的颗粒中的锂脱嵌的随机研究表明,在高超电势下,决定速率的步骤是阳离子在颗粒-电解质界面上的转移。在不形成导电复合电极的情况下进行尖晶石的(电)化学行为的测量。界面离子转移的动力学定义了在水环境中使用LiMn 2 O 4的电池放电速率的理论上限。






























 京公网安备 11010802027423号
京公网安备 11010802027423号