当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile synthesis of layered spinel ferrite from fly ash waste as a stable and active ketonisation catalyst
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.cej.2024.157797 Sasha Yang, Jinxing Gu, Binbin Qian, Jim Mensah, Adam F. Lee, Karen Wilson, Barbara Etschmann, Xiya Fang, Jisheng Ma, Qinfen Gu, Lian Zhang
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.cej.2024.157797 Sasha Yang, Jinxing Gu, Binbin Qian, Jim Mensah, Adam F. Lee, Karen Wilson, Barbara Etschmann, Xiya Fang, Jisheng Ma, Qinfen Gu, Lian Zhang
|
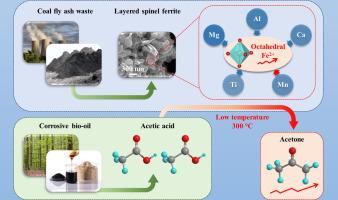
Spinel catalysts exhibit superior activity and structural stability across a wide range of catalytic reactions. Nevertheless, few studies have delved into the synthesis of spinels containing more than four metal cations, for which conventional syntheses from pure chemical precursors are costly and generate significant waste. Here we demonstrate a facile, rapid and scalable synthesis of layered spinel ferrite catalysts from fly ash waste that is otherwise detrimental to landfill ecosystems. The optimum waste-derived catalyst primarily comprised MgAl0.2Fe1.8O4, with a distorted structure due to the substitution of various cations (Ca2+, Mn2+, Mn3+, and Ti4+) at tetrahedral and/or octahedral iron sites, and demonstrates high activity (1.26 mmol⋅g−1⋅min−1) and stability (>100 h) for acetic acid ketonisation at a modest temperature (300 °C). Acidity measurements yield a corresponding turnover frequency of 2.21 min−1. Strong synergies are observed between the different metallic cations and octahedral Fe2+ species; XANES and in-situ DRIFTS indicate the latter is the primary active sites for ketonisation in fly ash-derived spinel ferrites, promoting both acetic acid adsorption as bidentate acetate and subsequent C–C coupling to acetone.
中文翻译:

从粉煤灰废料中轻松合成层状尖晶石铁氧体,作为稳定和活性的酮化催化剂
尖晶石催化剂在各种催化反应中表现出优异的活性和结构稳定性。然而,很少有研究深入研究含有四种以上金属阳离子的尖晶石的合成,为此,由纯化学前体进行的传统合成成本高昂,并产生大量浪费。在这里,我们展示了一种从粉煤灰废料中简单、快速和可扩展的分层尖晶石铁氧体催化剂合成的方法,否则会对垃圾填埋场生态系统有害。最佳的废物衍生催化剂主要由 MgAl0.2Fe1.8O4 组成,由于在四面体和/或八面体铁位点被各种阳离子(Ca2+、Mn2+、Mn3+ 和 Ti4+)取代,其结构扭曲,并表现出高活性 (1.26 mmol⋅g-1⋅min-1) 和稳定性 (>100 h) 在适度温度 (300 °C) 下进行乙酸酮化。酸度测量产生相应的周转频率为 2.21 min-1。在不同的金属阳离子和八面体 Fe2+ 物种之间观察到很强的协同作用;XANES 和原位漂移表明,后者是粉煤灰衍生的尖晶石铁氧体中酮化的主要活性位点,促进乙酸吸附为乙酸双齿酸盐,并随后促进 C-C 与丙酮的偶联。
更新日期:2024-11-19
中文翻译:

从粉煤灰废料中轻松合成层状尖晶石铁氧体,作为稳定和活性的酮化催化剂
尖晶石催化剂在各种催化反应中表现出优异的活性和结构稳定性。然而,很少有研究深入研究含有四种以上金属阳离子的尖晶石的合成,为此,由纯化学前体进行的传统合成成本高昂,并产生大量浪费。在这里,我们展示了一种从粉煤灰废料中简单、快速和可扩展的分层尖晶石铁氧体催化剂合成的方法,否则会对垃圾填埋场生态系统有害。最佳的废物衍生催化剂主要由 MgAl0.2Fe1.8O4 组成,由于在四面体和/或八面体铁位点被各种阳离子(Ca2+、Mn2+、Mn3+ 和 Ti4+)取代,其结构扭曲,并表现出高活性 (1.26 mmol⋅g-1⋅min-1) 和稳定性 (>100 h) 在适度温度 (300 °C) 下进行乙酸酮化。酸度测量产生相应的周转频率为 2.21 min-1。在不同的金属阳离子和八面体 Fe2+ 物种之间观察到很强的协同作用;XANES 和原位漂移表明,后者是粉煤灰衍生的尖晶石铁氧体中酮化的主要活性位点,促进乙酸吸附为乙酸双齿酸盐,并随后促进 C-C 与丙酮的偶联。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号