当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 capture performance of amine-functionalized amorphous SiO2-Al2O3 adsorbent: Insights into the support acidity
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.seppur.2024.130600 Xinlong Yan, Zhongyang Chen, Yingkun Zhu, Xiaoyan Hu, Guojun Kang, Xuehua Shen, Ling Liu, Shijian Lu, Mengqing Hu
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.seppur.2024.130600 Xinlong Yan, Zhongyang Chen, Yingkun Zhu, Xiaoyan Hu, Guojun Kang, Xuehua Shen, Ling Liu, Shijian Lu, Mengqing Hu
|
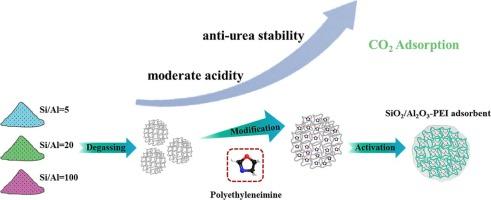
Amine-functionalized adsorbents possess considerable potential for CO2 capture due to their high selectivity and versatility across a range of applications. However, they are susceptible to CO2-induced chemical deactivation. Despite research efforts to synthesize supports with abundant acid sites to accommodate amines and enhance their stability, information remains sparse on how changes in surface acids impact the CO2 adsorption performance of the resulting adsorbents. In this context, we synthesized porous amorphous SiO2-Al2O3 with varying surface acidity, and impregnated with polyethylenimine (PEI). We then investigated the CO2 adsorption performance under different temperatures, regeneration atmospheres, and humidity levels. The results indicated an optimal adsorption temperature of 75 °C and a pre-treatment temperature of 140 °C. Under these conditions, the Si/Al = 20–60 sample demonstrated the highest capture capacity, approximately 142.6 mg/g. The Avrami model proved most suitable for fitting CO2 adsorption data across various adsorbents, providing an accurate assessment of the entire dynamic adsorption process. However, cycle stability tests revealed that Si/Al = 5–50 had the highest stability among the SiO2-Al2O3 adsorbents in both dry and humid conditions, due to its superior resistance to urea formation. Utilizing FT-IR, solid-state NMR, and XPS analysis, we discovered that the density of moderately strong Lewis acid sites on the surface of SiO2-Al2O3 plays a crucial role in resisting urea formation, as it induces the highest degree of cross-linking reaction between PEI and the porous supports. This breakthrough offers new insights into how the surface acidity of support materials influences the stability of solid amine adsorbents for CO2 capture.
中文翻译:

胺官能化无定形SiO2-Al2O3吸附剂的CO2捕集性能研究−支持酸度洞察
胺官能团化吸附剂由于其在一系列应用中的高选择性和多功能性,因此具有相当大的 CO2 捕获潜力。然而,它们容易受到 CO2 诱导的化学失活的影响。尽管研究努力合成具有丰富酸位点的载体以容纳胺并增强其稳定性,但关于表面酸的变化如何影响所得吸附剂的 CO2 吸附性能的信息仍然很少。在此背景下,我们合成了具有不同表面酸度的多孔无定形 SiO2-Al 2O3,并浸渍了聚乙烯亚胺 (PEI)。然后,我们研究了不同温度、再生气氛和湿度水平下的 CO2 吸附性能。结果表明,最佳吸附温度为 75 °C,前处理温度为 140 °C。 在这些条件下,Si/Al = 20–60 样品表现出最高的捕获容量,约为 142.6 mg/g。事实证明,Avrami 模型最适合拟合各种吸附剂的 CO2 吸附数据,从而准确评估整个动态吸附过程。然而,循环稳定性测试表明,Si/Al = 5–50 在 SiO 2-Al2O3 吸附剂中,由于其优异的抗尿素形成能力,在干燥和潮湿条件下均具有最高的稳定性。利用 FT-IR、固体 NMR 和 XPS 分析,我们发现 SiO2-Al 2O3 表面中等强度路易斯酸位点的密度在抵抗尿素形成方面起着至关重要的作用,因为它诱导 PEI 和多孔载体之间发生最高程度的交联反应。 这一突破为支持材料的表面酸性如何影响用于 CO2 捕获的固体胺吸附剂的稳定性提供了新的见解。
更新日期:2024-11-22
中文翻译:

胺官能化无定形SiO2-Al2O3吸附剂的CO2捕集性能研究−支持酸度洞察
胺官能团化吸附剂由于其在一系列应用中的高选择性和多功能性,因此具有相当大的 CO2 捕获潜力。然而,它们容易受到 CO2 诱导的化学失活的影响。尽管研究努力合成具有丰富酸位点的载体以容纳胺并增强其稳定性,但关于表面酸的变化如何影响所得吸附剂的 CO2 吸附性能的信息仍然很少。在此背景下,我们合成了具有不同表面酸度的多孔无定形 SiO2-Al 2O3,并浸渍了聚乙烯亚胺 (PEI)。然后,我们研究了不同温度、再生气氛和湿度水平下的 CO2 吸附性能。结果表明,最佳吸附温度为 75 °C,前处理温度为 140 °C。 在这些条件下,Si/Al = 20–60 样品表现出最高的捕获容量,约为 142.6 mg/g。事实证明,Avrami 模型最适合拟合各种吸附剂的 CO2 吸附数据,从而准确评估整个动态吸附过程。然而,循环稳定性测试表明,Si/Al = 5–50 在 SiO 2-Al2O3 吸附剂中,由于其优异的抗尿素形成能力,在干燥和潮湿条件下均具有最高的稳定性。利用 FT-IR、固体 NMR 和 XPS 分析,我们发现 SiO2-Al 2O3 表面中等强度路易斯酸位点的密度在抵抗尿素形成方面起着至关重要的作用,因为它诱导 PEI 和多孔载体之间发生最高程度的交联反应。 这一突破为支持材料的表面酸性如何影响用于 CO2 捕获的固体胺吸附剂的稳定性提供了新的见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号