当前位置:
X-MOL 学术
›
Electrochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pitfall on the interpretation of double layer capacitance increase after accelerated stress test of hydrogen evolution reaction on NiMo catalysts
Electrochimica Acta ( IF 5.5 ) Pub Date : 2024-11-17 , DOI: 10.1016/j.electacta.2024.145358 Abdul Majeed, Nicholas Hemmerling, Bastian J.M. Etzold
Electrochimica Acta ( IF 5.5 ) Pub Date : 2024-11-17 , DOI: 10.1016/j.electacta.2024.145358 Abdul Majeed, Nicholas Hemmerling, Bastian J.M. Etzold
|
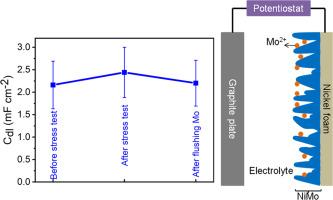
Critical investigation of methods used to screen the catalytic activity of different electrocatalysts is crucial for the development of water electrolysis technology. One of such protocols to justify the catalytic activity trend among different catalysts involves determining their electrochemically active surface area (ECSA) which is usually estimated from non-Faradic double layered capacitance (Cdl) of the catalyst material. Furthermore, catalytic current normalized with ECSA is frequently used to gain insight into the intrinsic activity of a catalyst. Since not all the metallic catalysts are highly stable under the electrolysis conditions, it is highly important, though rarely explored, to investigate whether or not the adsorption/desorption of leached/dissolved multivalent metal ions influences the measurement of non-Faradic Cdl. Here, we explore the possible influence of Mo leached from NiMo alloy on the measured Cdl of NiMo. Using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS), Cdl of NiMo alloy was measured before and after carrying out long term (chronopotentiometric and CV cycling) hydrogen evolution reaction (HER) in alkaline conditions. After extended HER testing, we observe a notable rise (∼22 % averaging all our experiments) in the Cdl of NiMo alloy when measured with traditional methods. Interestingly, only 8 % of this increase can be attributed to an expansion in surface area. We hypothesize that the majority of the increase in Cdl stems from the higher amount of charges stored through leached multivalent ions, which accumulate within surface cavities.
中文翻译:

NiMo 催化剂析氢反应加速应力试验后双电层电容增加解释的陷阱
对用于筛选不同电催化剂催化活性的方法进行批判性研究对于水电解技术的发展至关重要。证明不同催化剂之间催化活性趋势的方案之一涉及确定它们的电化学活性表面积 (ECSA),这通常根据催化剂材料的非法拉第双电层电容 (Cdl) 估算。此外,用 ECSA 归一化的催化电流经常用于深入了解催化剂的本征活性。由于并非所有金属催化剂在电解条件下都高度稳定,因此研究浸出/溶解的多价金属离子的吸附/解吸是否影响非法拉第克 Cdl 的测量非常重要,尽管很少进行探索。在这里,我们探讨了从 NiMo 合金中浸出的 Mo 对 NiMo 的测量 Cdl 的可能影响。使用循环伏安法 (CV) 和电化学阻抗谱 (EIS),在碱性条件下进行长期(计时电位和 CV 循环)析氢反应 (HER) 之前和之后测量了 NiMo 合金的 Cdl。在扩展的 HER 测试后,我们观察到用传统方法测量时,NiMo 合金的 Cdl 显着升高(我们所有实验的平均 ∼22%)。有趣的是,这种增加中只有 8% 可以归因于表面积的扩大。我们假设 Cdl 的大部分增加源于通过浸出的多价离子储存的大量电荷,这些电荷在表面腔内积累。
更新日期:2024-11-21
中文翻译:

NiMo 催化剂析氢反应加速应力试验后双电层电容增加解释的陷阱
对用于筛选不同电催化剂催化活性的方法进行批判性研究对于水电解技术的发展至关重要。证明不同催化剂之间催化活性趋势的方案之一涉及确定它们的电化学活性表面积 (ECSA),这通常根据催化剂材料的非法拉第双电层电容 (Cdl) 估算。此外,用 ECSA 归一化的催化电流经常用于深入了解催化剂的本征活性。由于并非所有金属催化剂在电解条件下都高度稳定,因此研究浸出/溶解的多价金属离子的吸附/解吸是否影响非法拉第克 Cdl 的测量非常重要,尽管很少进行探索。在这里,我们探讨了从 NiMo 合金中浸出的 Mo 对 NiMo 的测量 Cdl 的可能影响。使用循环伏安法 (CV) 和电化学阻抗谱 (EIS),在碱性条件下进行长期(计时电位和 CV 循环)析氢反应 (HER) 之前和之后测量了 NiMo 合金的 Cdl。在扩展的 HER 测试后,我们观察到用传统方法测量时,NiMo 合金的 Cdl 显着升高(我们所有实验的平均 ∼22%)。有趣的是,这种增加中只有 8% 可以归因于表面积的扩大。我们假设 Cdl 的大部分增加源于通过浸出的多价离子储存的大量电荷,这些电荷在表面腔内积累。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号