当前位置:
X-MOL 学术
›
Cell Host Microbe
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A trivalent mucosal vaccine encoding phylogenetically inferred ancestral RBD sequences confers pan-Sarbecovirus protection in mice
Cell Host & Microbe ( IF 20.6 ) Pub Date : 2024-11-18 , DOI: 10.1016/j.chom.2024.10.016 James Brett Case, Shilpa Sanapala, Carly Dillen, Victoria Rhodes, Christian Zmasek, Taras M. Chicz, Charlotte E. Switzer, Suzanne M. Scheaffer, George Georgiev, Catherine Jacob-Dolan, Blake M. Hauser, Déborah Carolina Carvalho Dos Anjos, Lucas J. Adams, Nadia Soudani, Chieh-Yu Liang, Baoling Ying, Ryan P. McNamara, Richard H. Scheuermann, Adrianus C.M. Boon, Daved H. Fremont, Michael S. Diamond
Cell Host & Microbe ( IF 20.6 ) Pub Date : 2024-11-18 , DOI: 10.1016/j.chom.2024.10.016 James Brett Case, Shilpa Sanapala, Carly Dillen, Victoria Rhodes, Christian Zmasek, Taras M. Chicz, Charlotte E. Switzer, Suzanne M. Scheaffer, George Georgiev, Catherine Jacob-Dolan, Blake M. Hauser, Déborah Carolina Carvalho Dos Anjos, Lucas J. Adams, Nadia Soudani, Chieh-Yu Liang, Baoling Ying, Ryan P. McNamara, Richard H. Scheuermann, Adrianus C.M. Boon, Daved H. Fremont, Michael S. Diamond
|
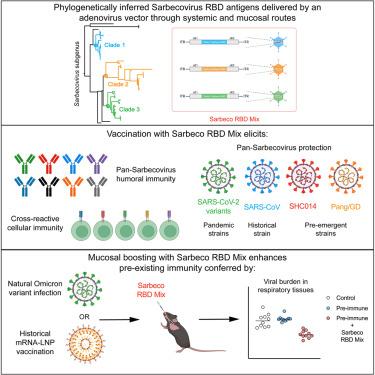
The continued emergence of SARS-CoV-2 variants and the threat of future Sarbecovirus zoonoses have spurred the design of vaccines that can induce broad immunity against multiple coronaviruses. Here, we use computational methods to infer ancestral phylogenetic reconstructions of receptor binding domain (RBD) sequences across multiple Sarbecovirus clades and incorporate them into a multivalent adenoviral-vectored vaccine. Mice immunized with this pan-Sarbecovirus vaccine are protected in the upper and lower respiratory tracts against infection by historical and contemporary SARS-CoV-2 variants, SARS-CoV, and pre-emergent SHC014 and Pangolin/GD coronavirus strains. Using genetic and immunological approaches, we demonstrate that vaccine-induced protection unexpectedly is conferred principally by CD4+ and CD8+ T cell-mediated anamnestic responses. Importantly, prior mRNA vaccination or SARS-CoV-2 respiratory infection does not alter the efficacy of the mucosally delivered pan-Sarbecovirus vaccine. These data highlight the promise of a phylogenetic approach for antigen and vaccine design against existing and pre-emergent Sarbecoviruses with pandemic potential.
中文翻译:

编码系统发育推断的祖先 RBD 序列的三价粘膜疫苗在小鼠中赋予泛 Sarbecovirus 保护
SARS-CoV-2 变体的持续出现和未来 Sarbecovirus 人畜共患病的威胁刺激了可以诱导对多种冠状病毒的广泛免疫力的疫苗的设计。在这里,我们使用计算方法来推断跨多个 Sarbecovirus 分支的受体结合域 (RBD) 序列的祖先系统发育重建,并将它们整合到多价腺病毒载体疫苗中。用这种泛 Sarbecovirus 疫苗免疫的小鼠在上呼吸道和下呼吸道受到保护,免受历史和当代 SARS-CoV-2 变体、SARS-CoV 以及出苗前 SHC014 和穿山甲/GD 冠状病毒株的感染。使用遗传学和免疫学方法,我们证明疫苗诱导的保护主要由 CD4 + 和 CD8 + T 细胞介导的遗忘反应意外赋予。重要的是,先前的 mRNA 疫苗接种或 SARS-CoV-2 呼吸道感染不会改变粘膜递送的泛 Sarbecovirus 疫苗的功效。这些数据突出了针对具有大流行潜力的现有和出苗前 Sarbecoviruses 的抗原和疫苗设计系统发育方法的前景。
更新日期:2024-11-18
中文翻译:

编码系统发育推断的祖先 RBD 序列的三价粘膜疫苗在小鼠中赋予泛 Sarbecovirus 保护
SARS-CoV-2 变体的持续出现和未来 Sarbecovirus 人畜共患病的威胁刺激了可以诱导对多种冠状病毒的广泛免疫力的疫苗的设计。在这里,我们使用计算方法来推断跨多个 Sarbecovirus 分支的受体结合域 (RBD) 序列的祖先系统发育重建,并将它们整合到多价腺病毒载体疫苗中。用这种泛 Sarbecovirus 疫苗免疫的小鼠在上呼吸道和下呼吸道受到保护,免受历史和当代 SARS-CoV-2 变体、SARS-CoV 以及出苗前 SHC014 和穿山甲/GD 冠状病毒株的感染。使用遗传学和免疫学方法,我们证明疫苗诱导的保护主要由 CD4 + 和 CD8 + T 细胞介导的遗忘反应意外赋予。重要的是,先前的 mRNA 疫苗接种或 SARS-CoV-2 呼吸道感染不会改变粘膜递送的泛 Sarbecovirus 疫苗的功效。这些数据突出了针对具有大流行潜力的现有和出苗前 Sarbecoviruses 的抗原和疫苗设计系统发育方法的前景。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号