当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preclinical development of lyophilized self-replicating RNA vaccines for COVID-19 and malaria with improved long-term thermostability
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-17 , DOI: 10.1016/j.jconrel.2024.11.023 Gaurav K. Gulati, Adrian C. Simpson, Zachary MacMillen, Kyle Krieger, Shibbu Sharma, Jesse H. Erasmus, Steven G. Reed, James W. Davie, Marion Avril, Amit P. Khandhar
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-17 , DOI: 10.1016/j.jconrel.2024.11.023 Gaurav K. Gulati, Adrian C. Simpson, Zachary MacMillen, Kyle Krieger, Shibbu Sharma, Jesse H. Erasmus, Steven G. Reed, James W. Davie, Marion Avril, Amit P. Khandhar
|
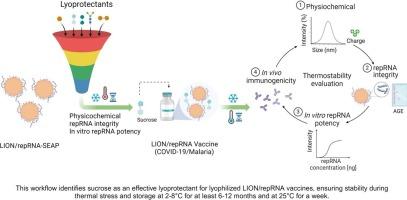
Messenger RNA (mRNA) vaccines against COVID-19 have demonstrated high efficacy and rapid deployment capability to target emerging infectious diseases. However, the need for ultra-low temperature storage made the distribution of LNP/mRNA vaccines to regions with limited resources impractical. This study explores the use of lyophilization to enhance the stability of self-replicating mRNA (repRNA) vaccines, allowing for their storage at non-freezing temperatures such as 2–8 °C or room temperature (25 °C). We lyophilized repRNA molecules complexed to a novel cationic emulsion delivery system, LION™, with different sugar-based lyoprotectants to identify candidates that provided the best vaccine integrity and effectiveness after being thermally stressed. For screening, we used repRNA encoding the reporter protein secreted embryonic alkaline phosphatase (SEAP) and for proof-of-concept, we used repRNA vaccines encoding SARS-CoV-2 full-length spike (WA-1 isolate) or full-length surface protein circumsporozoite (CS) of Plasmodium yoelii (Py). We found that lyophilization of LION/repRNA with sucrose provided the best colloidal stability, preserved in vitro expression, and induced equivalent antigen-specific antibody responses in mice compared to freshly prepared liquid LION/repRNA. Furthermore, lyophilized vaccines were stable for at least one week at 25 °C and at least one year at 2–8 °C. The cumulative analysis of stability-determining physicochemical data, in vitro potency, and in vivo immunogenicity in mice enabled the selection of a lead lyophilized composition containing 10 % w /v sucrose as the lyoprotectant. The data presented here provide a foundation for the clinical evaluation of next-generation thermostable repRNA vaccines that will enable more equitable vaccine access globally.
中文翻译:

用于 COVID-19 和疟疾的冻干自复制 RNA 疫苗的临床前开发,具有更好的长期热稳定性
针对 COVID-19 的信使 RNA (mRNA) 疫苗已显示出针对新发传染病的高效和快速部署能力。然而,对超低温储存的需求使得将 LNP/mRNA 疫苗分发到资源有限的地区变得不切实际。本研究探讨了使用冻干来提高自我复制 mRNA (repRNA) 疫苗的稳定性,使其能够在非冷冻温度下储存,例如 2-8 °C 或室温 (25 °C)。我们将 repRNA 分子与新型阳离子乳剂递送系统 LION™ 复合,使用不同的糖基冻干保护剂,以确定在热应激后提供最佳疫苗完整性和有效性的候选者。为了进行筛选,我们使用了编码报告蛋白分泌的胚胎碱性磷酸酶 (SEAP) 的 repRNA,为了进行概念验证,我们使用了编码 SARS-CoV-2 全长刺突(WA-1 分离物)或全长表面蛋白圆子孢子 (CS) 的 repRNA 疫苗约氏疟原虫 (Py)。我们发现,与新鲜制备的液体 LION/repRNA 相比,用蔗糖冻干 LION/repRNA 提供了最佳的胶体稳定性,保留了体外表达,并在小鼠中诱导了等效的抗原特异性抗体反应。此外,冻干疫苗在 25 °C 下至少可稳定保存一周,在 2-8 °C 下至少可稳定保存一年。 对小鼠稳定性决定理化数据、体外效力和体内免疫原性的累积分析能够选择含有 10% w/v 蔗糖作为冻干剂的铅冻干组合物。 此处提供的数据为下一代热稳定 repRNA 疫苗的临床评估奠定了基础,这将使全球疫苗获得更公平。
更新日期:2024-11-17
中文翻译:

用于 COVID-19 和疟疾的冻干自复制 RNA 疫苗的临床前开发,具有更好的长期热稳定性
针对 COVID-19 的信使 RNA (mRNA) 疫苗已显示出针对新发传染病的高效和快速部署能力。然而,对超低温储存的需求使得将 LNP/mRNA 疫苗分发到资源有限的地区变得不切实际。本研究探讨了使用冻干来提高自我复制 mRNA (repRNA) 疫苗的稳定性,使其能够在非冷冻温度下储存,例如 2-8 °C 或室温 (25 °C)。我们将 repRNA 分子与新型阳离子乳剂递送系统 LION™ 复合,使用不同的糖基冻干保护剂,以确定在热应激后提供最佳疫苗完整性和有效性的候选者。为了进行筛选,我们使用了编码报告蛋白分泌的胚胎碱性磷酸酶 (SEAP) 的 repRNA,为了进行概念验证,我们使用了编码 SARS-CoV-2 全长刺突(WA-1 分离物)或全长表面蛋白圆子孢子 (CS) 的 repRNA 疫苗约氏疟原虫 (Py)。我们发现,与新鲜制备的液体 LION/repRNA 相比,用蔗糖冻干 LION/repRNA 提供了最佳的胶体稳定性,保留了体外表达,并在小鼠中诱导了等效的抗原特异性抗体反应。此外,冻干疫苗在 25 °C 下至少可稳定保存一周,在 2-8 °C 下至少可稳定保存一年。 对小鼠稳定性决定理化数据、体外效力和体内免疫原性的累积分析能够选择含有 10% w/v 蔗糖作为冻干剂的铅冻干组合物。 此处提供的数据为下一代热稳定 repRNA 疫苗的临床评估奠定了基础,这将使全球疫苗获得更公平。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号