当前位置:
X-MOL 学术
›
Sep. Purif. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction kinetic modeling of carbon dioxide desorption in aqueous amine solutions
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-16 , DOI: 10.1016/j.seppur.2024.130578 Rui-Qi Jia, Shuang Liang, Zhi-Yuan Xue, Guang-Wen Chu, Liang-Liang Zhang, Jian-Feng Chen
Separation and Purification Technology ( IF 8.1 ) Pub Date : 2024-11-16 , DOI: 10.1016/j.seppur.2024.130578 Rui-Qi Jia, Shuang Liang, Zhi-Yuan Xue, Guang-Wen Chu, Liang-Liang Zhang, Jian-Feng Chen
|
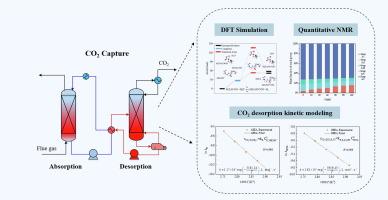
CO2 desorption is a critical process for chemical absorption carbon capture approach, and the reaction kinetics is an important basis for the design and scale-up of desorption process. Here, kinetic modeling of CO2 desorption in primary/secondary and tertiary amines was developed for N-(2-aminoethyl) ethanolamine (AEEA) and N,N-diethylethanolamine (DEEA), which are typical primary/secondary and tertiary amines, respectively. With the assumption that the protonated amine and carbamate or bicarbonate have the same concentration at the same moment, the desorption kinetic modeling of the two types of amines can be expressed by a pseudo-second-order equation. Quantitative speciation of the components in the absorbent was performed by 1H and 13C NMR spectra, which supported the model assumptions. Furthermore, quantum chemical calculation was conducted to reveal the reaction mechanism during CO2 desorption. Model reliability was verified by N-methyl diethanolamine (MDEA) desorption data. The Arrhenius kinetic equations for the desorption of AEEA and DEEA were determined, and the activation energies were 43.08 and 49.21 kJ/mol, respectively. The validated kinetic model of CO2 desorption is promising to provide fundamental parameters for the design and optimization of regeneration units for CO2 capture.
中文翻译:

二氧化碳在胺水溶液中解吸的反应动力学模型
CO2 脱附是化学吸收碳捕集方法的关键过程,反应动力学是脱附过程设计和放大生产的重要基础。本文针对 N-(2-氨基乙基)乙醇胺 (AEEA) 和 N,N-二乙基乙醇胺 (DEEA) 开发了 CO2 在伯/仲胺和叔胺中解吸的动力学模型,它们分别是典型的伯/仲胺和叔胺。假设质子化胺和氨基甲酸酯或碳酸氢盐在同一时刻具有相同的浓度,两种胺的解吸动力学模型可以用准二阶方程表示。通过 1H 和 13C NMR 波谱对吸收剂中的成分进行定量形态分析,这支持了模型假设。此外,还进行了量子化学计算,以揭示 CO2 解吸过程中的反应机理。通过 N-甲基二乙醇胺 (MDEA) 解吸数据验证了模型可靠性。确定了 AEEA 和 DEEA 解吸的 Arrhenius 动力学方程,活化能分别为 43.08 和 49.21 kJ/mol。经过验证的 CO2 解吸动力学模型有望为 CO2 捕获再生装置的设计和优化提供基本参数。
更新日期:2024-11-16
中文翻译:

二氧化碳在胺水溶液中解吸的反应动力学模型
CO2 脱附是化学吸收碳捕集方法的关键过程,反应动力学是脱附过程设计和放大生产的重要基础。本文针对 N-(2-氨基乙基)乙醇胺 (AEEA) 和 N,N-二乙基乙醇胺 (DEEA) 开发了 CO2 在伯/仲胺和叔胺中解吸的动力学模型,它们分别是典型的伯/仲胺和叔胺。假设质子化胺和氨基甲酸酯或碳酸氢盐在同一时刻具有相同的浓度,两种胺的解吸动力学模型可以用准二阶方程表示。通过 1H 和 13C NMR 波谱对吸收剂中的成分进行定量形态分析,这支持了模型假设。此外,还进行了量子化学计算,以揭示 CO2 解吸过程中的反应机理。通过 N-甲基二乙醇胺 (MDEA) 解吸数据验证了模型可靠性。确定了 AEEA 和 DEEA 解吸的 Arrhenius 动力学方程,活化能分别为 43.08 和 49.21 kJ/mol。经过验证的 CO2 解吸动力学模型有望为 CO2 捕获再生装置的设计和优化提供基本参数。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号