当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In vitro-in vivo correlation (IVIVC) development for long-acting injectable drug products based on poly(lactide-co-glycolide)
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.jconrel.2024.11.021 Yan Wang, Andrew Otte, Haesun Park, Kinam Park
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-19 , DOI: 10.1016/j.jconrel.2024.11.021 Yan Wang, Andrew Otte, Haesun Park, Kinam Park
|
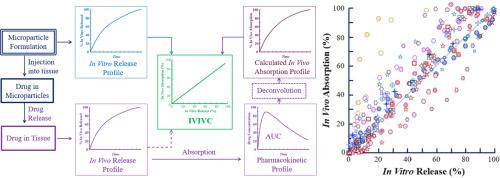
In vitro-in vivo correlation (IVIVC), linking in vitro drug release to in vivo drug release or in vivo drug absorption, has been explored chiefly for oral extended-release dosage forms. Currently, there are no official guidelines on IVIVC development for non-oral drug delivery systems. Recently, many long-acting injectable (LAI) formulations based on poly(lactide-co-glycolide) (PLGA) have been developed to deliver various drugs, ranging from small molecules to peptides and proteins, for up to 6 months. The circumstances involved in the LAI formulations are drastically different from those in oral formulations, which generally deliver drugs for a maximum of 24 h. This article examines 37 IVIVC studies of PLGA microparticle formulations available in the literature. Understanding and establishing an IVIVC of LAI formulations requires more than merely plotting the percentage in vitro drug release against the percentage in vivo absorption. In vivo drug absorption (or release) should be measured to provide a complete pharmacokinetic profile when feasible. Accelerated in vitro release methods need to be respective of the real-time measurements by sharing the same release mechanism. Obtaining meaningful IVIVCs with predictive capability will be highly useful for future regulatory actions and for developing generic and new formulations.
中文翻译:

基于聚丙交酯-共乙交酯的长效注射药物产品的体外体内相关 (IVIVC) 开发
体外-体内相关性 (IVIVC) 将体外药物释放与体内药物释放或体内药物吸收联系起来,主要用于口服缓释剂型。目前,没有关于非口服给药系统 IVIVC 开发的官方指南。最近,已经开发了许多基于聚丙交酯-共乙交酯 (PLGA) 的长效注射剂 (LAI) 制剂,用于输送各种药物,从小分子到肽和蛋白质,持续时间长达 6 个月。LAI 制剂所涉及的情况与口服制剂中涉及的情况截然不同,口服制剂通常最多给药 24 小时。本文研究了文献中可用的 PLGA 微粒制剂的 37 项 IVIVC 研究。了解和建立 LAI 制剂的 IVIVC 需要的不仅仅是绘制体外药物释放百分比与体内吸收百分比的关系图。在可行的情况下,应测量体内药物吸收(或释放)以提供完整的药代动力学特征。加速体外释放方法需要通过共享相同的释放机制来与实时测量相一致。获得具有预测能力的有意义的 IVIVC 将对未来的监管行动以及开发仿制药和新配方非常有用。
更新日期:2024-11-19
中文翻译:

基于聚丙交酯-共乙交酯的长效注射药物产品的体外体内相关 (IVIVC) 开发
体外-体内相关性 (IVIVC) 将体外药物释放与体内药物释放或体内药物吸收联系起来,主要用于口服缓释剂型。目前,没有关于非口服给药系统 IVIVC 开发的官方指南。最近,已经开发了许多基于聚丙交酯-共乙交酯 (PLGA) 的长效注射剂 (LAI) 制剂,用于输送各种药物,从小分子到肽和蛋白质,持续时间长达 6 个月。LAI 制剂所涉及的情况与口服制剂中涉及的情况截然不同,口服制剂通常最多给药 24 小时。本文研究了文献中可用的 PLGA 微粒制剂的 37 项 IVIVC 研究。了解和建立 LAI 制剂的 IVIVC 需要的不仅仅是绘制体外药物释放百分比与体内吸收百分比的关系图。在可行的情况下,应测量体内药物吸收(或释放)以提供完整的药代动力学特征。加速体外释放方法需要通过共享相同的释放机制来与实时测量相一致。获得具有预测能力的有意义的 IVIVC 将对未来的监管行动以及开发仿制药和新配方非常有用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号