当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of Enzymes and Their Key Action Sites for Histamine Degradation in Mulberry Fruit Wine by Lactiplantibacillus plantarum
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.jafc.4c06615 Xiaowei Chen, Wenshan Luo, Xinyi Ye, Yujuan Xu, Jijun Wu, Yuanshan Yu, Jian Peng, Lina Cheng, Lu Li
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.jafc.4c06615 Xiaowei Chen, Wenshan Luo, Xinyi Ye, Yujuan Xu, Jijun Wu, Yuanshan Yu, Jian Peng, Lina Cheng, Lu Li
|
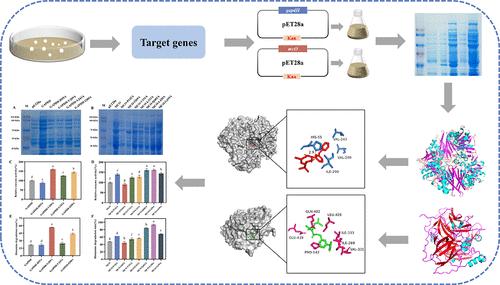
In this study, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and multicopper oxidase (MCO) of Lactiplantibacillus plantarum W155 with histamine degradation ability were expressed. The mulberry fruit wine (MFW) histamine degradation abilities of GAPDH and MCO were 20.81% and 37.67%, respectively. Compared with the control group, the MFW treated by GAPDH showed higher total phenolic (1.17 g GAE/L) and total flavonoid (0.31 g RE/L) contents, while MFW treated by MCO presented similar total phenolic (1.00 g GAE/L) and total flavonoid (0.29 g RE/L) concentrations. Furthermore, the optimal pH and temperature of GAPDH were 6.0 and 40 °C, respectively, while the optimal pH and temperature of MCO were 3.0 and 50 °C, respectively. Meanwhile, the key action sites for histamine degradation of GAPDH and MCO were minded via homology modeling, molecular docking, and site-directed mutagenesis. Val209 and Ile290 were confirmed as the key action sites for GAPDH, while Qln402 and Leu420 were the pivotal action sites for MCO. Above findings indicated that both GAPDH and MCO of L. plantarum W155 could be used to control the histamine of MFW, and the key action sites of these two enzymes could be used as targets for their subsequent modification.
中文翻译:

植物乳植杆菌鉴定桑果酒中组胺降解的酶及其关键作用位点
本研究表达了具有组胺降解能力的植物乳植杆菌 W155 的甘油醛-3-磷酸脱氢酶 (GAPDH) 和多铜氧化酶 (MCO)。GAPDH 和 MCO 对桑果酒 (MFW) 组胺的降解能力分别为 20.81% 和 37.67%。与对照组相比,GAPDH 处理的 MFW 表现出更高的总酚酸 (1.17 g GAE/L) 和总黄酮 (0.31 g RE/L) 含量,而 MCO 处理的 MFW 表现出相似的总酚 (1.00 g GAE/L) 和总黄酮 (0.29 g RE/L) 浓度。此外,GAPDH 的最适 pH 值和温度分别为 6.0 和 40 °C,而 MCO 的最适 pH 值和温度分别为 3.0 °C 和 50 °C。同时,通过同源建模、分子对接和定点诱变来关注 GAPDH 和 MCO 组胺降解的关键作用位点。Val209 和 Ile290 被证实是 GAPDH 的关键作用位点,而 Qln402 和 Leu420 是 MCO 的关键作用位点。以上结果表明,植物乳杆菌 W155 的 GAPDH 和 MCO 均可用于控制 MFW 的组胺,这两种酶的关键作用位点可作为其后续修饰的靶点。
更新日期:2024-11-14
中文翻译:

植物乳植杆菌鉴定桑果酒中组胺降解的酶及其关键作用位点
本研究表达了具有组胺降解能力的植物乳植杆菌 W155 的甘油醛-3-磷酸脱氢酶 (GAPDH) 和多铜氧化酶 (MCO)。GAPDH 和 MCO 对桑果酒 (MFW) 组胺的降解能力分别为 20.81% 和 37.67%。与对照组相比,GAPDH 处理的 MFW 表现出更高的总酚酸 (1.17 g GAE/L) 和总黄酮 (0.31 g RE/L) 含量,而 MCO 处理的 MFW 表现出相似的总酚 (1.00 g GAE/L) 和总黄酮 (0.29 g RE/L) 浓度。此外,GAPDH 的最适 pH 值和温度分别为 6.0 和 40 °C,而 MCO 的最适 pH 值和温度分别为 3.0 °C 和 50 °C。同时,通过同源建模、分子对接和定点诱变来关注 GAPDH 和 MCO 组胺降解的关键作用位点。Val209 和 Ile290 被证实是 GAPDH 的关键作用位点,而 Qln402 和 Leu420 是 MCO 的关键作用位点。以上结果表明,植物乳杆菌 W155 的 GAPDH 和 MCO 均可用于控制 MFW 的组胺,这两种酶的关键作用位点可作为其后续修饰的靶点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号