当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A personalized vaccine combining immunogenic cell death-induced cells and nanosized antigens for enhanced antitumor immunity
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-13 , DOI: 10.1016/j.jconrel.2024.10.060 Ying Yang, Peng Zheng, Biao Duan, Ying Yang, Xiao Zheng, Weiran Li, Qingwen Liu, Yongmao Hu, Yanbing Ma
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-13 , DOI: 10.1016/j.jconrel.2024.10.060 Ying Yang, Peng Zheng, Biao Duan, Ying Yang, Xiao Zheng, Weiran Li, Qingwen Liu, Yongmao Hu, Yanbing Ma
|
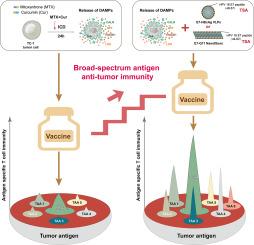
The tumor vaccine aims to activate the immune system, promote antitumor cellular responses, and restore immune recognition and clearance of tumor cells. However, the low immunogenicity and heterogeneity of tumor antigens, along with immunosuppressive mechanisms, severely hinder tumor vaccines from achieving an efficient and sustained antitumor effect. Herein, we developed a combined vaccine strategy that utilizes immunogenic cell death (ICD) to elicit a broad spectrum of antigen-specific responses in a whole-cell-based manner. Additionally, we introduced nanosized antigens to intensify immune responses targeting a key tumor antigen. The combination of mitoxantrone (MTX) and curcumin (Cur) optimized ICD properties in TC-1 tumor cells, as evidenced by increased release of “find me” signals, such as HMGB1 and ATP, and enhanced exposure of the “eat me” signal, CALR, compared to either MTX or Cur alone. Correspondingly, the ICD cells induced by the combination produced more significant antitumor effects in vivo . Furthermore, the ICD cells in combination with E7-HBcAg VLPs or E7-Q11 nanofibers induced more intense effector cell responses to the antigen included in the nanovaccines, as well as a broad spectrum of antigens provided by tumor cells, and significantly suppressed the growth of established tumors compared with either ICD cells, VLPs, or nanofibers alone. In conclusion, the combination of ICD cells and nanosized antigens produced synergistic antitumor effects and elicited robust and comprehensive antitumor immunity, presenting an attractive strategy for developing personalized tumor vaccines.
中文翻译:

一种结合免疫原性细胞死亡诱导细胞和纳米抗原的个性化疫苗,用于增强抗肿瘤免疫力
肿瘤疫苗旨在激活免疫系统,促进抗肿瘤细胞反应,恢复肿瘤细胞的免疫识别和清除。然而,肿瘤抗原的低免疫原性和异质性以及免疫抑制机制严重阻碍了肿瘤疫苗实现有效和持续的抗肿瘤效果。在此,我们开发了一种联合疫苗策略,利用免疫原性细胞死亡 (ICD) 以基于全细胞的方式引发广泛的抗原特异性反应。此外,我们引入了纳米大小的抗原来增强针对关键肿瘤抗原的免疫反应。米托蒽醌 (MTX) 和姜黄素 (Cur) 的组合优化了 TC-1 肿瘤细胞中的 ICD 特性,与单独的 MTX 或 Cur 相比,“找到我”信号(如 HMGB1 和 ATP)的释放增加,以及“吃我”信号 CALR 的暴露增加。相应地,该组合诱导的 ICD 细胞在体内产生更显著的抗肿瘤作用。此外,ICD 细胞与 E7-HBcAg VLP 或 E7-Q11 纳米纤维联合诱导了更强烈的效应细胞对纳米疫苗中包含的抗原以及肿瘤细胞提供的广谱抗原的反应,并且与单独的 ICD 细胞、VLP 或纳米纤维相比,显着抑制了已建立的肿瘤的生长。总之,ICD 细胞和纳米抗原的组合产生了协同抗肿瘤作用,并引发了强大而全面的抗肿瘤免疫,为开发个性化肿瘤疫苗提供了一种有吸引力的策略。
更新日期:2024-11-13
中文翻译:

一种结合免疫原性细胞死亡诱导细胞和纳米抗原的个性化疫苗,用于增强抗肿瘤免疫力
肿瘤疫苗旨在激活免疫系统,促进抗肿瘤细胞反应,恢复肿瘤细胞的免疫识别和清除。然而,肿瘤抗原的低免疫原性和异质性以及免疫抑制机制严重阻碍了肿瘤疫苗实现有效和持续的抗肿瘤效果。在此,我们开发了一种联合疫苗策略,利用免疫原性细胞死亡 (ICD) 以基于全细胞的方式引发广泛的抗原特异性反应。此外,我们引入了纳米大小的抗原来增强针对关键肿瘤抗原的免疫反应。米托蒽醌 (MTX) 和姜黄素 (Cur) 的组合优化了 TC-1 肿瘤细胞中的 ICD 特性,与单独的 MTX 或 Cur 相比,“找到我”信号(如 HMGB1 和 ATP)的释放增加,以及“吃我”信号 CALR 的暴露增加。相应地,该组合诱导的 ICD 细胞在体内产生更显著的抗肿瘤作用。此外,ICD 细胞与 E7-HBcAg VLP 或 E7-Q11 纳米纤维联合诱导了更强烈的效应细胞对纳米疫苗中包含的抗原以及肿瘤细胞提供的广谱抗原的反应,并且与单独的 ICD 细胞、VLP 或纳米纤维相比,显着抑制了已建立的肿瘤的生长。总之,ICD 细胞和纳米抗原的组合产生了协同抗肿瘤作用,并引发了强大而全面的抗肿瘤免疫,为开发个性化肿瘤疫苗提供了一种有吸引力的策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号