当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-catalyzed synthesis of N-substituted 3-acylpyrroles from enaminones and vinylene carbonate
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4qo01816h Jian-Bo Ma, Yi-Mai Yin, Xing-Mei Hu, Bi-Na Shao, Kun Huang, Qing-Sheng Zhao, Sheng-Jiao Yan
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-12 , DOI: 10.1039/d4qo01816h Jian-Bo Ma, Yi-Mai Yin, Xing-Mei Hu, Bi-Na Shao, Kun Huang, Qing-Sheng Zhao, Sheng-Jiao Yan
|
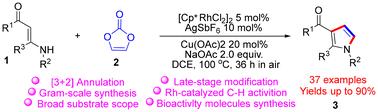
Herein, we present a protocol for constructing N-substituted 3-carbonylpyrroles (NSCPRs) through the reaction of vinylene carbonate with enaminone building blocks, facilitated by Rh(III)-catalyzed [3 + 2] annulation. This reaction enabled the straightforward and efficient construction of NSCPRs and their drug analogs. Specifically, we developed a cascade Rh(III)-catalyzed C–H activation/[3 + 2] annulation reaction for enaminones and internal vinylene carbonate, catalyzed by [Cp*RhCl2]2 and oxidized with Cu(OAc)2, AgSbF6 and air. This method allowed for the efficient synthesis of highly functionalized NSCPRs with good yields. The reaction proceeded through C–H activation, alkene insertion, decarboxylation and reductive elimination, ketone-enol tautomerization, 1,2-addition, and dehydration. This protocol demonstrates excellent functional group tolerance, a broad substrate scope, and scalability for gram-scale synthesis, showcasing its applicability to drug molecules and highlighting its utility in organic synthesis and pharmaceutical chemistry.
中文翻译:

铑催化从烯胺和碳酸乙烯酯合成 N-取代的 3-酰基吡咯
在此,我们提出了一种通过碳酸乙烯酯与烯胺酮构建单元反应构建 N-取代的 3-羰基吡咯 (NSCPR) 的方案,由 Rh(III) 催化的 [3 + 2] 环化促进。该反应使 NSCPR 及其药物类似物的构建变得简单而高效。具体来说,我们开发了一种级联 Rh(III) 催化的烯胺类化合物和内部碳酸乙烯酯的 C-H 活化/[3 + 2] 环化反应,由 [Cp*RhCl2]2 催化并用 Cu(OAc)2、AgSbF6 和空气氧化。该方法允许高效合成高度功能化的 NSCPR,产量高。反应通过 C-H 活化、烯烃插入、脱羧和还原消除、酮-烯醇互变异构化、1,2-加成和脱水进行。该方案表现出优异的官能团耐受性、广泛的底物范围和克级合成的可扩展性,展示了其对药物分子的适用性,并突出了其在有机合成和药物化学中的实用性。
更新日期:2024-11-12
中文翻译:

铑催化从烯胺和碳酸乙烯酯合成 N-取代的 3-酰基吡咯
在此,我们提出了一种通过碳酸乙烯酯与烯胺酮构建单元反应构建 N-取代的 3-羰基吡咯 (NSCPR) 的方案,由 Rh(III) 催化的 [3 + 2] 环化促进。该反应使 NSCPR 及其药物类似物的构建变得简单而高效。具体来说,我们开发了一种级联 Rh(III) 催化的烯胺类化合物和内部碳酸乙烯酯的 C-H 活化/[3 + 2] 环化反应,由 [Cp*RhCl2]2 催化并用 Cu(OAc)2、AgSbF6 和空气氧化。该方法允许高效合成高度功能化的 NSCPR,产量高。反应通过 C-H 活化、烯烃插入、脱羧和还原消除、酮-烯醇互变异构化、1,2-加成和脱水进行。该方案表现出优异的官能团耐受性、广泛的底物范围和克级合成的可扩展性,展示了其对药物分子的适用性,并突出了其在有机合成和药物化学中的实用性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号