当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulating Coordination Environment of Cobalt-Based Spinel Octahedral Metal Sites to Boost Metal–Oxygen Bond Covalency for Reversible Lithium–Oxygen Batteries
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-13 , DOI: 10.1021/acssuschemeng.4c06092 Yu Pan, Anjun Hu, Ruizhe Xu, Jingze Chen, Borui Yang, Ting Li, Kun Li, Yuanjian Li, Zhi Wei Seh, Jianping Long
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-13 , DOI: 10.1021/acssuschemeng.4c06092 Yu Pan, Anjun Hu, Ruizhe Xu, Jingze Chen, Borui Yang, Ting Li, Kun Li, Yuanjian Li, Zhi Wei Seh, Jianping Long
|
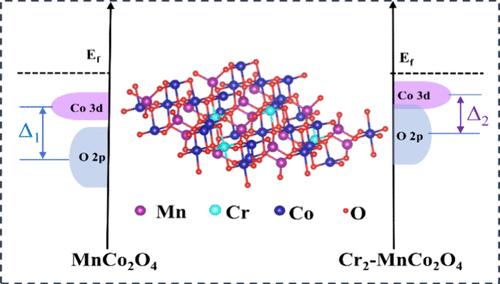
The intrinsic catalytic activity of conventional spinel electrocatalysts hinders their electrocatalytic outcomes in lithium–oxygen batteries (LOBs), despite their appeal due to compositional variety and structural adaptability. In this work, we reveal that the electrocatalytic activities of these catalysts can be inherently enhanced by modulating metal–oxygen (M–O) bond covalency interactions through the introduction of the Cr element into the MnCo2O4 octahedral sites (MnCr0.5Co1.5O4). The introduction of Cr3+ directly alters the coordination structure of Co octahedral sites, which increases the Co3+–O distance and reduces the lattice symmetry, resulting in enhanced covalency interactions of the M–O bond. Computational analysis supports the effectiveness of Cr in altering the electronic structure of the active site, narrowing the energy gap between Co 3d and O 2p orbitals, evidencing the enhancement of the M–O covalency. In addition, this increased M–O covalency accelerates charge transfer in oxygen-related reactions, thereby facilitating the reversible formation and decomposition of the discharge products in LOBs. As a proof of concept, the MnCr0.5Co1.5O4 catalyzed LOBs exhibit a large discharge capacity of 16 388.3 mAh g–1 and maintain stability over 329 cycles. This work paves the way for the progression of reversible LOBs by manipulating the coordination structure of the spinel catalysts.
中文翻译:

调控钴基尖晶石八面体金属位点的配位环境以提高可逆锂氧电池的金属-氧键共价性
传统尖晶石电催化剂的本征催化活性阻碍了它们在锂氧电池 (LOB) 中的电催化结果,尽管它们由于成分多样性和结构适应性而具有吸引力。在这项工作中,我们揭示了通过将 Cr 元素引入 MnCo2O4 八面体位点 (MnCr0.5Co1.5O4) 中,通过调节金属-氧 (M-O) 键共价相互作用,可以从本质上增强这些催化剂的电催化活性。Cr3+ 的引入直接改变了 Co 八面体位点的配位结构,这增加了 Co3+–O 的距离并降低了晶格对称性,从而增强了 M–O 键的共价相互作用。计算分析支持 Cr 在改变活性位点的电子结构、缩小 Co 3d 和 O 2p 轨道之间的能隙方面的有效性,证明了 M-O 共价的增强。此外,这种增加的 M-O 共价性加速了氧相关反应中的电荷转移,从而促进了 LOB 中放电产物的可逆形成和分解。作为概念验证,MnCr0.5Co1.5O4 催化的 LOB 表现出 16 388.3 mAh g–1 的大放电容量,并在 329 次循环中保持稳定性。这项工作通过操纵尖晶石催化剂的配位结构,为可逆 LOBs 的进展铺平了道路。
更新日期:2024-11-13
中文翻译:

调控钴基尖晶石八面体金属位点的配位环境以提高可逆锂氧电池的金属-氧键共价性
传统尖晶石电催化剂的本征催化活性阻碍了它们在锂氧电池 (LOB) 中的电催化结果,尽管它们由于成分多样性和结构适应性而具有吸引力。在这项工作中,我们揭示了通过将 Cr 元素引入 MnCo2O4 八面体位点 (MnCr0.5Co1.5O4) 中,通过调节金属-氧 (M-O) 键共价相互作用,可以从本质上增强这些催化剂的电催化活性。Cr3+ 的引入直接改变了 Co 八面体位点的配位结构,这增加了 Co3+–O 的距离并降低了晶格对称性,从而增强了 M–O 键的共价相互作用。计算分析支持 Cr 在改变活性位点的电子结构、缩小 Co 3d 和 O 2p 轨道之间的能隙方面的有效性,证明了 M-O 共价的增强。此外,这种增加的 M-O 共价性加速了氧相关反应中的电荷转移,从而促进了 LOB 中放电产物的可逆形成和分解。作为概念验证,MnCr0.5Co1.5O4 催化的 LOB 表现出 16 388.3 mAh g–1 的大放电容量,并在 329 次循环中保持稳定性。这项工作通过操纵尖晶石催化剂的配位结构,为可逆 LOBs 的进展铺平了道路。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号