当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxy-pyridinium Ylides Mediated 1,4-Pyridyl/Aryl Translocation
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03656 Yaping Tang, Meirong Huang, Zichun Yan, Shengbiao Tang, Xinhao Zhang, Jiangtao Sun
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03656 Yaping Tang, Meirong Huang, Zichun Yan, Shengbiao Tang, Xinhao Zhang, Jiangtao Sun
|
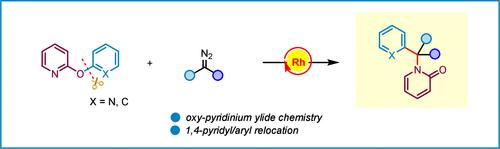
Molecular rearrangement via carbene transfer is a powerful tool to access molecular diversity. Herein, we describe an efficient approach to selective pyridyl/aryl relocation via a rhodium-catalyzed aminoarylation of diazo compounds, providing a promising strategy to access ortho-pyridyl N-alkylated pyridone scaffolds in a single operation. This reaction features the novel reactivity of oxy-pyridinium ylide, rhodium-associated five-membered transition state, and 1,4-pyridyl/aryl relocation. A computational study discloses the initial oxy-pyridinium ylide formation, keto–enol tautomerization, and 1,4-pyridyl migration to complete the whole rearrangement.
中文翻译:

氧-吡啶酰基化物介导的 1,4-吡啶基/芳基易位
通过卡宾转移进行分子重排是获得分子多样性的有力工具。在本文中,我们描述了一种通过铑催化的重氮化合物氨基芳基化进行选择性吡啶基/芳基重定位的有效方法,提供了一种在单次操作中进入邻吡啶基 N-烷基化吡啶酮支架的有前途的策略。该反应具有氧-吡啶酰化物的新型反应性、铑相关的五元过渡态和 1,4-吡啶基/芳基重定位。一项计算研究揭示了初始氧-吡啶酰亚铵形成、酮-烯醇互变异构化和 1,4-吡啶基迁移,以完成整个重排。
更新日期:2024-11-11
中文翻译:

氧-吡啶酰基化物介导的 1,4-吡啶基/芳基易位
通过卡宾转移进行分子重排是获得分子多样性的有力工具。在本文中,我们描述了一种通过铑催化的重氮化合物氨基芳基化进行选择性吡啶基/芳基重定位的有效方法,提供了一种在单次操作中进入邻吡啶基 N-烷基化吡啶酮支架的有前途的策略。该反应具有氧-吡啶酰化物的新型反应性、铑相关的五元过渡态和 1,4-吡啶基/芳基重定位。一项计算研究揭示了初始氧-吡啶酰亚铵形成、酮-烯醇互变异构化和 1,4-吡啶基迁移,以完成整个重排。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号