当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronically Activated CuFeO2 via Graphene Oxide for Highly Efficient CO2 Conversion into Long-Chain Hydrocarbons
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-11 , DOI: 10.1021/acssuschemeng.4c05474 Zhiyuan Zhang, Yuxue Wei, Jun Yu, Mingyang Ren, Chunqiu Zhao, Fang Chen, Chenghua Zhang, Yong Jiang, Lisheng Guo, Song Sun
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-11 , DOI: 10.1021/acssuschemeng.4c05474 Zhiyuan Zhang, Yuxue Wei, Jun Yu, Mingyang Ren, Chunqiu Zhao, Fang Chen, Chenghua Zhang, Yong Jiang, Lisheng Guo, Song Sun
|
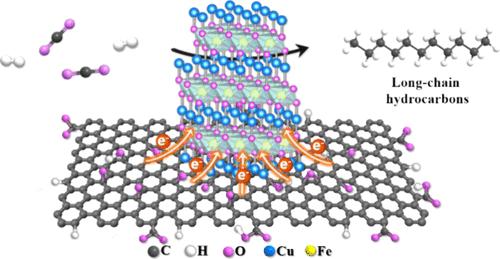
Utilizing CO2 as a feedstock to produce valuable chemicals and fuels provides a potential alternative to traditional methods reducing dependence on petroleum resources. It has been acknowledged that incorporating the reverse water gas shift (RWGS) and Fischer–Tropsch synthesis (FTS) active phase in a tandem system is crucial for optimizing the efficiency of CO2 hydrogenation. The formation of RWGS and FTS active phases in catalysts often involves creating an electron-rich local environment. In this study, graphene oxide (GO)-supported CuFeO2 was in situ synthesized under hydrothermal conditions (CuFeO2/GO-H), achieving an intimate interface and thus facilitating the electron transfer from GO to CuFeO2. The CuFeO2/GO-H exhibits a CO2 conversion of 36.8% and a C5+ selectivity of 66.0%, which are 1.6 and 1.4 times higher than those of pristine CuFeO2. The augmented CO2 hydrogenation performance of CuFeO2/GO-H is credited to the electron transfer facilitated by the cation−π interaction between CuFeO2 and surface oxygen-containing groups on GO. The electron transfer from GO to CuFeO2 not only boosts the adsorption of CO2 and CO but also promotes the reduction and carburization of CuFeO2 to form more small-sized Cu-χ-Fe5C2 active sites. In addition, the layer structure of GO with certain defects ensures a close proximity between the supported active phases, facilitating the formation of small-sized Cu and χ-Fe5C2 nanoparticles, as well as the exposure of Cu-χ-Fe5C2 interfaces. The small particle size and well-integrated Cu and χ-Fe5C2 in CuFeO2/GO-H enhance its CO2 conversion and C5+ selectivity by providing more active sites and reducing the kinetic barriers for C–C coupling. This study aligns with efforts to design bifunctional catalysts by emphasizing the electron transfer process in optimizing the rate of CO2 hydrogenation.
中文翻译:

通过氧化石墨烯实现电子活化 CuFeO2 将 CO2 高效转化为长链碳氢化合物
利用 CO2 作为原料来生产有价值的化学品和燃料,为减少对石油资源的依赖提供了传统方法的潜在替代方案。众所周知,在串联系统中加入反向水气变换 (RWGS) 和 Fischer-Tropsch 合成 (FTS) 活性相对于优化 CO2 加氢效率至关重要。在催化剂中形成 RWGS 和 FTS 活性相通常涉及创造富含电子的局部环境。在本研究中,在水热条件下原位合成了氧化石墨烯 (GO) 负载的 CuFeO2 (CuFeO2/GO-H),实现了紧密界面,从而促进了电子从 GO 到 CuFeO2 的转移。CuFeO2/GO-H 的 CO2 转化率为 36.8%,C5+ 选择性为 66.0%,分别是原始 CuFeO2 的 1.6 倍和 1.4 倍。CuFeO2/GO-H 增强的 CO2 加氢性能归功于 CuFeO2 与 GO 上表面含氧基团之间的阳离子-π 相互作用促进的电子转移。从 GO 到 CuFeO2 的电子转移不仅促进了 CO2 和 CO 的吸附,还促进了 CuFeO2 的还原和渗碳,形成了更多小尺寸的 Cu-χ-Fe5C2 活性位点。此外,具有某些缺陷的 GO 的层结构确保了负载的活性相之间的紧密接近,有利于形成小尺寸 Cu 和 χ-Fe5C2 纳米颗粒,以及 Cu-χ-Fe5C2 界面的暴露。 CuFeO2/GO-H 中的小粒径和良好整合的 Cu 和 χ-Fe5C2 通过提供更多的活性位点和减少 C-C 耦合的动力学障碍,提高了其 CO2 转化率和 C5+ 选择性。本研究与设计双功能催化剂的努力相一致,强调电子转移过程在优化 CO2 加氢速率方面的作用。
更新日期:2024-11-12
中文翻译:

通过氧化石墨烯实现电子活化 CuFeO2 将 CO2 高效转化为长链碳氢化合物
利用 CO2 作为原料来生产有价值的化学品和燃料,为减少对石油资源的依赖提供了传统方法的潜在替代方案。众所周知,在串联系统中加入反向水气变换 (RWGS) 和 Fischer-Tropsch 合成 (FTS) 活性相对于优化 CO2 加氢效率至关重要。在催化剂中形成 RWGS 和 FTS 活性相通常涉及创造富含电子的局部环境。在本研究中,在水热条件下原位合成了氧化石墨烯 (GO) 负载的 CuFeO2 (CuFeO2/GO-H),实现了紧密界面,从而促进了电子从 GO 到 CuFeO2 的转移。CuFeO2/GO-H 的 CO2 转化率为 36.8%,C5+ 选择性为 66.0%,分别是原始 CuFeO2 的 1.6 倍和 1.4 倍。CuFeO2/GO-H 增强的 CO2 加氢性能归功于 CuFeO2 与 GO 上表面含氧基团之间的阳离子-π 相互作用促进的电子转移。从 GO 到 CuFeO2 的电子转移不仅促进了 CO2 和 CO 的吸附,还促进了 CuFeO2 的还原和渗碳,形成了更多小尺寸的 Cu-χ-Fe5C2 活性位点。此外,具有某些缺陷的 GO 的层结构确保了负载的活性相之间的紧密接近,有利于形成小尺寸 Cu 和 χ-Fe5C2 纳米颗粒,以及 Cu-χ-Fe5C2 界面的暴露。 CuFeO2/GO-H 中的小粒径和良好整合的 Cu 和 χ-Fe5C2 通过提供更多的活性位点和减少 C-C 耦合的动力学障碍,提高了其 CO2 转化率和 C5+ 选择性。本研究与设计双功能催化剂的努力相一致,强调电子转移过程在优化 CO2 加氢速率方面的作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号