当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Implemention of Innovative Process Analytical Technologies to Characterize Critical Quality Attributes of Co‐Formulated Monoclonal Antibody Products
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-11-08 , DOI: 10.1002/bit.28881 Apurva Godbole, Lyufei Chen, Jay Desai, Smita Raghava, Richard Ruzanski, Bhumit Patel, Emmanuel Appiah‐Amponsah, Hanzhou Feng
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-11-08 , DOI: 10.1002/bit.28881 Apurva Godbole, Lyufei Chen, Jay Desai, Smita Raghava, Richard Ruzanski, Bhumit Patel, Emmanuel Appiah‐Amponsah, Hanzhou Feng
|
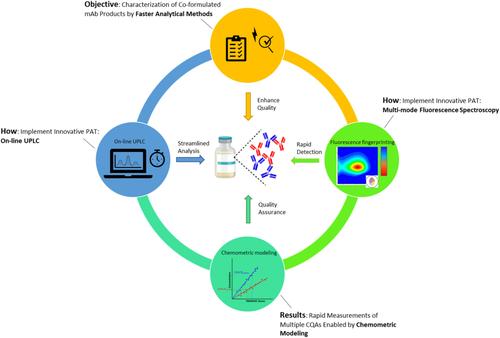
Characterizing co‐formulated monoclonal antibodies (mAbs) poses significant challenges in the pharmaceutical industry. Due to the high structural similarity of the mAbs, traditional analytical methods, compounded by the lengthy method development process, hinder product development and manufacturing efficiency. There is increasing critical need in the pharmaceutical industry to streamline analytical approaches, minimizing time and resources, ensuring a rapid clinical entry and cost‐effective manufacturing. This study investigates the application of process analytical technologies (PAT) to address such challenges. Our investigation introduces two complementary technologies, on‐line ultra‐performance liquid chromatography (online UPLC) and multimode fluorescence spectroscopy (MMFS), as potential PAT tools tailored for characterizing critical quality attributes (CQA) in co‐formulated mAb products. Specifically, the CQAs under evaluation include the total protein concentration of the mAbs within the co‐formulation and the ratio of mAb A to mAb B. Online UPLC enables direct and automated measurement of the CQAs through physical separation, while MMFS determines them in a non‐destructive and more swift manner based on chemometric modeling. We demonstrate these technologies' comparable performance to conventional methods, alongside substantial benefits such as reduced analytical turnaround time and decreased laboratory efforts. Ultimately, integrating them as innovative PAT tools expedites the delivery of therapeutic solutions to patients and enhances manufacturing efficiency, aligning with the imperative for swift translation of scientific discoveries into clinical benefits.
中文翻译:

实施创新的过程分析技术来表征共配制单克隆抗体产品的关键质量属性
表征共配制的单克隆抗体 (mAb) 对制药行业提出了重大挑战。由于 mAb 的结构高度相似,传统的分析方法加上漫长的方法开发过程,阻碍了产品开发和生产效率。制药行业越来越迫切地需要简化分析方法,最大限度地减少时间和资源,确保快速进入临床和经济高效的生产。本研究调查了过程分析技术 (PAT) 在应对此类挑战中的应用。我们的研究介绍了两种互补技术,在线超高效液相色谱(在线 UPLC)和多模式荧光光谱 (MMFS),作为潜在的 PAT 工具,专为表征共配制的 mAb 产品中的关键质量属性 (CQA) 而量身定制。具体而言,所评估的CQA包括共制剂中mAb的总蛋白浓度以及mAb A与mAbB的比率。在线UPLC支持通过物理分离直接和自动测量CQA,而MMFS则基于化学计量学建模以非破坏性和更快速的方式确定它们。我们证明了这些技术与传统方法的性能相当,以及诸如缩短分析周转时间和减少实验室工作量等显著优势。最终,将它们整合为创新的 PAT 工具可以加快向患者提供治疗解决方案并提高生产效率,从而满足将科学发现迅速转化为临床益处的必要性。
更新日期:2024-11-08
中文翻译:

实施创新的过程分析技术来表征共配制单克隆抗体产品的关键质量属性
表征共配制的单克隆抗体 (mAb) 对制药行业提出了重大挑战。由于 mAb 的结构高度相似,传统的分析方法加上漫长的方法开发过程,阻碍了产品开发和生产效率。制药行业越来越迫切地需要简化分析方法,最大限度地减少时间和资源,确保快速进入临床和经济高效的生产。本研究调查了过程分析技术 (PAT) 在应对此类挑战中的应用。我们的研究介绍了两种互补技术,在线超高效液相色谱(在线 UPLC)和多模式荧光光谱 (MMFS),作为潜在的 PAT 工具,专为表征共配制的 mAb 产品中的关键质量属性 (CQA) 而量身定制。具体而言,所评估的CQA包括共制剂中mAb的总蛋白浓度以及mAb A与mAbB的比率。在线UPLC支持通过物理分离直接和自动测量CQA,而MMFS则基于化学计量学建模以非破坏性和更快速的方式确定它们。我们证明了这些技术与传统方法的性能相当,以及诸如缩短分析周转时间和减少实验室工作量等显著优势。最终,将它们整合为创新的 PAT 工具可以加快向患者提供治疗解决方案并提高生产效率,从而满足将科学发现迅速转化为临床益处的必要性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号