当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfur Heterocyclic Quinone Cathodes for Rechargeable Magnesium Batteries: Discharge Voltage, Cycling Stability, and Reaction Reversibility Improved by Sulfur Substitution
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-08 , DOI: 10.1021/acssuschemeng.4c06991 Hongda Gui, Donggang Tao, Yudi Tang, Yuliang Cao, Fei Xu
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-11-08 , DOI: 10.1021/acssuschemeng.4c06991 Hongda Gui, Donggang Tao, Yudi Tang, Yuliang Cao, Fei Xu
|
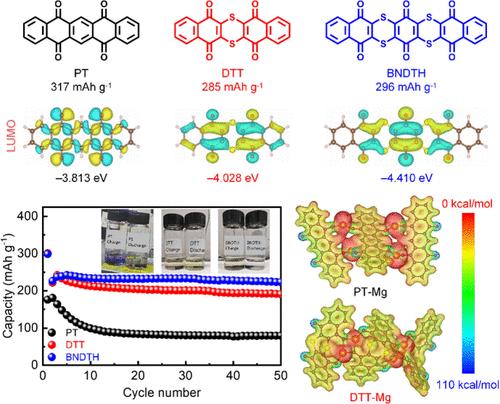
Rechargeable magnesium batteries have the potential for large-scale energy-storage applications, but traditional inorganic cathodes suffer from inferior performance and insufficient selections. Organic conjugated carbonyl compounds are promising cathode materials with a delocalized negative charge, reversible carbonyl enolization, and wide designability. Herein, sulfur heterocyclic quinones of dibenzo[b,i]thianthrene-5,7,12,14-tetraone (DTT) and benzo[b]naphtho[2′,3′:5,6][1,4]dithiino[2,3-i]thianthrene-5,7,9,14,16,18-hexone (BNDTH) are prepared and investigated as cathode materials for rechargeable magnesium batteries and compared with normal quinone of 5,7,12,14-pentacenetetrone (PT). DTT and BNDTH show higher redox potentials, higher magnesium storage capacities, and better cycling stabilities than PT. Mechanism study and theoretical computation reveal that the adjacent carbonyls connected with sulfur atoms in DTT and BNDTH coordinate with bivalent magnesium cations better than rigid PT via conformation change, leading to better reaction reversibility. DTT and BNDTH have lower LUMO energy levels and thus higher redox potentials than PT. DTT and BNDTH show lower solubilities in the electrolytes than PT and result in a higher cycling stability. The comparative study herein would provide scientific insights into the rational design of organic cathode materials suitable for reversible and stable storage reactions of bivalent magnesium cations.
中文翻译:

用于可充电镁电池的硫杂环醌阴极:通过硫取代提高放电电压、循环稳定性和反应可逆性
可充电镁电池具有大规模储能应用的潜力,但传统的无机阴极性能较差且选择不足。有机共轭羰基化合物是很有前途的正极材料,具有离域负电荷、可逆的羰基烯醇化和广泛的可设计性。在此,制备了二苯并[b,i]硫代-5,7,12,14-四酮(DTT)和苯并[b]萘酚[2′,3′:5,6][1,4]二硫代[2,3-i]硫代-5,7,9,14,16,18-己酮(BNDTH)的硫杂环醌,并将其作为可充电镁电池的正极材料进行研究,并与5,7,12,14-五乙烯酮(PT)的正常醌进行了比较。DTT 和 BNDTH 显示出比 PT 更高的氧化还原电位、更高的镁储存能力和更好的循环稳定性。机理研究和理论计算表明,DTT 和 BNDTH 中与硫原子连接的相邻羰基化合物通过构象变化比刚性 PT 更好地与二价镁阳离子配位,导致更好的反应可逆性。DTT 和 BNDTH 具有较低的 LUMO 能级,因此比 PT 具有更高的氧化还原电位。DTT 和 BNDTH 在电解质中的溶解度低于 PT,因此具有更高的循环稳定性。本文的比较研究将为适用于二价镁阳离子可逆和稳定储存反应的有机正极材料的合理设计提供科学见解。
更新日期:2024-11-09
中文翻译:

用于可充电镁电池的硫杂环醌阴极:通过硫取代提高放电电压、循环稳定性和反应可逆性
可充电镁电池具有大规模储能应用的潜力,但传统的无机阴极性能较差且选择不足。有机共轭羰基化合物是很有前途的正极材料,具有离域负电荷、可逆的羰基烯醇化和广泛的可设计性。在此,制备了二苯并[b,i]硫代-5,7,12,14-四酮(DTT)和苯并[b]萘酚[2′,3′:5,6][1,4]二硫代[2,3-i]硫代-5,7,9,14,16,18-己酮(BNDTH)的硫杂环醌,并将其作为可充电镁电池的正极材料进行研究,并与5,7,12,14-五乙烯酮(PT)的正常醌进行了比较。DTT 和 BNDTH 显示出比 PT 更高的氧化还原电位、更高的镁储存能力和更好的循环稳定性。机理研究和理论计算表明,DTT 和 BNDTH 中与硫原子连接的相邻羰基化合物通过构象变化比刚性 PT 更好地与二价镁阳离子配位,导致更好的反应可逆性。DTT 和 BNDTH 具有较低的 LUMO 能级,因此比 PT 具有更高的氧化还原电位。DTT 和 BNDTH 在电解质中的溶解度低于 PT,因此具有更高的循环稳定性。本文的比较研究将为适用于二价镁阳离子可逆和稳定储存反应的有机正极材料的合理设计提供科学见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号