当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Commercial Manufacturing Process for Vepdegestrant, an Orally Bioavailable PROTAC Estrogen Receptor Degrader for the Treatment of Breast Cancer
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-05 , DOI: 10.1021/acs.oprd.4c00362 Steve Avery, Jamie M. Buske, Doris Chen, Herman Chen, Xin Chen, Andrew R. Davidson, Jean-Nicolas Desrosiers, Hanqing Dong, Noalle Fellah, David F. Fernández, John Grosso, Lu Han, Teri Hochdorfer, Amber M. Johnson, Brian P. Jones, Maciej Kalinowski, Katherine D. Launer-Felty, Jorge Lopez, Teresa Makowski, Carolyn Mastriano, Truong N. Nguyen, Nitinchandra D. Patel, Zhihui Peng, Tyler Potter, Robert P. Pritchard, Anil M. Rane, Max Reeve, Margaret C. Richins, Chase A. Salazar, John J. Salisbury, Robert Simpson, Liza Tabshey, Erin J. Tweed, Paul G. Wahome, Nancy Walsh-Sayles, Jordan A. Willie, Ethan Wood
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-05 , DOI: 10.1021/acs.oprd.4c00362 Steve Avery, Jamie M. Buske, Doris Chen, Herman Chen, Xin Chen, Andrew R. Davidson, Jean-Nicolas Desrosiers, Hanqing Dong, Noalle Fellah, David F. Fernández, John Grosso, Lu Han, Teri Hochdorfer, Amber M. Johnson, Brian P. Jones, Maciej Kalinowski, Katherine D. Launer-Felty, Jorge Lopez, Teresa Makowski, Carolyn Mastriano, Truong N. Nguyen, Nitinchandra D. Patel, Zhihui Peng, Tyler Potter, Robert P. Pritchard, Anil M. Rane, Max Reeve, Margaret C. Richins, Chase A. Salazar, John J. Salisbury, Robert Simpson, Liza Tabshey, Erin J. Tweed, Paul G. Wahome, Nancy Walsh-Sayles, Jordan A. Willie, Ethan Wood
|
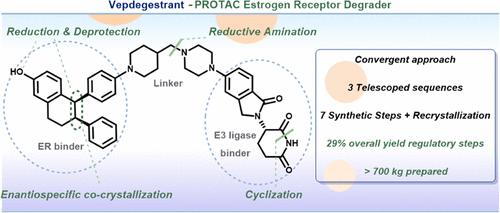
A commercial process for vepdegestrant (1), the most advanced PROTAC protein degrader in human clinical trials, has been developed to support clinical and commercial needs. The process features an efficient convergent synthetic strategy through the final reductive amination of two advanced chiral intermediates, as well as several highly efficient telescoped processes and robust crystallization for purity control. The final commercial process of vepdegestrant (1) consists of seven proposed regulatory GMP steps with five isolations in an overall yield of 29%.
中文翻译:

Vepdegestrant(一种用于治疗乳腺癌的口服生物可利用的 PROTAC 雌激素受体降解剂)的商业化生产工艺的开发
vepdegestrant (1) 是人体临床试验中最先进的 PROTAC 蛋白降解剂,已开发出一种商业工艺,以支持临床和商业需求。该工艺通过两种高级手性中间体的最终还原胺化,以及几种高效的伸缩工艺和稳健的结晶来控制纯度,采用高效的收敛合成策略。vepdegestrant (1) 的最终商业工艺包括 7 个拟议的监管 GMP 步骤和 5 个分离,总产量为 29%。
更新日期:2024-11-05
中文翻译:

Vepdegestrant(一种用于治疗乳腺癌的口服生物可利用的 PROTAC 雌激素受体降解剂)的商业化生产工艺的开发
vepdegestrant (1) 是人体临床试验中最先进的 PROTAC 蛋白降解剂,已开发出一种商业工艺,以支持临床和商业需求。该工艺通过两种高级手性中间体的最终还原胺化,以及几种高效的伸缩工艺和稳健的结晶来控制纯度,采用高效的收敛合成策略。vepdegestrant (1) 的最终商业工艺包括 7 个拟议的监管 GMP 步骤和 5 个分离,总产量为 29%。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号