Diabetologia ( IF 8.4 ) Pub Date : 2024-10-30 , DOI: 10.1007/s00125-024-06290-6 Pernille E. Hostrup, Tobias Schmidt, Simon B. Hellsten, Rebekka H. Gerwig, Joachim Størling, Jesper Johannesen, Karolina Sulek, Morten Hostrup, Henrik U. Andersen, Karsten Buschard, Yasmin Hamid, Flemming Pociot
|
Aims/hypothesis
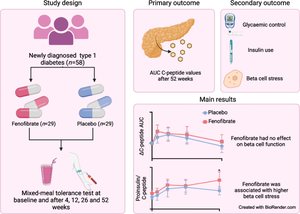
Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, shows some promise in alleviating beta cell stress and preserving beta cell function in preclinical studies of type 1 diabetes. The aim of this phase 2, placebo-controlled, double-blinded, randomised clinical trial was to investigate the efficacy and safety of fenofibrate in adults and adolescents with newly diagnosed type 1 diabetes.
Methods
We enrolled 58 individuals (aged 16 to 40 years old) with newly diagnosed type 1 diabetes and randomised them to daily oral treatment with fenofibrate 160 mg or placebo for 52 weeks (in a block design with a block size of 4, assigned in a 1:1 ratio). Our primary outcome was change in beta cell function after 52 weeks of treatment, assessed by AUC for C-peptide levels following a 2 h mixed-meal tolerance test. Secondary outcomes included glycaemic control (assessed by HbA1c and continuous glucose monitoring), daily insulin use, and proinsulin/C-peptide (PI/C) ratio as a marker of beta cell stress. We assessed outcome measures before and after 4, 12, 26 and 52 weeks of treatment. Blinding was maintained for participants, their healthcare providers and all staff involved in handling outcome samples and assessment.
Results
The statistical analyses for the primary outcome included 56 participants (n=27 in the fenofibrate group, after two withdrawals, and n=29 in the placebo group). We found no significant differences between the groups in either 2 h C-peptide levels (mean difference of 0.08 nmol/l [95% CI −0.05, 0.23]), insulin use or glycaemic control after 52 weeks of treatment. On the contrary, the fenofibrate group showed a higher PI/C ratio at week 52 compared with placebo (mean difference of 0.024 [95% CI 0.000, 0.048], p<0.05). Blood lipidome analysis revealed that fenofibrate repressed pathways involved in sphingolipid metabolism and signalling at week 52 compared with placebo. The 52 week intervention evoked few adverse events and no serious adverse events. Follow-up in vitro experiments in human pancreatic islets demonstrated a stress-inducing effect of fenofibrate.
Conclusions/interpretation
Contrary to the beneficial effects of fenofibrate found in preclinical studies, this longitudinal, randomised, placebo-controlled trial does not support the use of fenofibrate for preserving beta cell function in individuals with newly diagnosed type 1 diabetes.
Trial registration
EudraCT number: 2019-004434-41
Funding
This study was funded by the Sehested Hansens Foundation.
Graphical Abstract
中文翻译:

非诺贝特对新诊断的 1 型糖尿病成人和青少年残余 β 细胞功能的影响:一项随机临床试验
目标/假设
非诺贝特是一种过氧化物酶体增殖物激活的受体 α 激动剂,在 1 型糖尿病的临床前研究中显示出一些缓解 β 细胞应激和保持 β 细胞功能的前景。这项 2 期、安慰剂对照、双盲、随机临床试验的目的是研究非诺贝特在新诊断的 1 型糖尿病成人和青少年中的疗效和安全性。
方法
我们招募了 58 名新诊断的 1 型糖尿病患者(年龄 16 至 40 岁),并将他们随机分配到每日口服 160 mg 非诺贝特或安慰剂治疗 52 周(采用区组设计,区组大小为 4,以 1:1 的比例分配)。我们的主要结局是治疗 52 周后 β 细胞功能的变化,在 2 小时混合膳食耐受性试验后通过 AUC 评估 C 肽水平。次要结局包括血糖控制 (通过 HbA1c 和连续血糖监测评估)、每日胰岛素使用和胰岛素原/C 肽 (PI/C) 比率作为 β 细胞应激的标志物。我们评估了治疗 4 、 12 、 26 和 52 周前后的结局指标。对参与者、他们的医疗保健提供者和所有参与处理结果样本和评估的工作人员保持盲法。
结果
主要结局的统计分析包括 56 名参与者 (非诺贝特组 n=27,两次停药后,安慰剂组 n=29)。我们发现两组之间在 2 h C 肽水平 (平均差异为 0.08 nmol/l [95% CI -0.05, 0.23])、胰岛素使用或治疗 52 周后血糖控制方面均无显著差异。相反,与安慰剂组相比,非诺贝特组在第 52 周时显示出更高的 PI/C 比率 (平均差 0.024 [95% CI 0.000, 0.048],p<0.05)。血脂组分析显示,与安慰剂相比,非诺贝特在第 52 周抑制了参与鞘脂代谢和信号传导的通路。52 周的干预很少引起不良事件,也没有严重的不良事件。在人胰岛的后续体外实验表明非诺贝特具有应激诱导作用。
结论/解释
与临床前研究中发现的非诺贝特的有益作用相反,这项纵向、随机、安慰剂对照试验不支持使用非诺贝特来维持新诊断的 1 型糖尿病患者的 β 细胞功能。
试用注册
EudraCT编号:2019-004434-41
资金
这项研究由 Sehested Hansens 基金会资助。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号