当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anti-Fas Antibody Conjugated Nanoparticles Enhancing the Antitumor Effect of Camptothecin by Activating the Fas–FasL Apoptotic Pathway
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2016-10-26 00:00:00 , DOI: 10.1021/acsami.6b09760 Hongliang Yu 1 , Jian He , Qian Lu 1 , Da Huo 1 , Shanmei Yuan 1 , Zhengyang Zhou , Peipei Xu , Yong Hu 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2016-10-26 00:00:00 , DOI: 10.1021/acsami.6b09760 Hongliang Yu 1 , Jian He , Qian Lu 1 , Da Huo 1 , Shanmei Yuan 1 , Zhengyang Zhou , Peipei Xu , Yong Hu 1
Affiliation
|
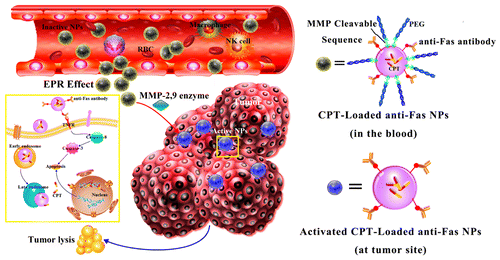
Emerging evidence suggest that the introduction of Fas ligand (FasL) can enhance the Fas-dependent apoptosis and induce durable immune responses against tumor. However, selective triggering of apoptosis in tumor cells while sparing normal cells remains a great challenge for the application of FasL-based therapeutic strategies. Herein, smart nanoparticles (NPs) with a sandwich structure were fabricated. These NPs consist of a matrix metalloproteinase (MMP) cleavable PEG outer layer, an anti-Fas antibody middle layer, and a camptothecin (CPT)-loaded inner core. They could accumulate at a tumor site by the enhanced permeability and retention (EPR) effect. The removable PEG layer protects the cytotoxic anti-Fas antibody from premature contact with normal tissues, thus avoiding the unexpected lethal side effect before they reach the tumor site. Due to the high level of MMP expressed by tumor cells inside the tumor tissue, these NPs would shed their PEG layers, resulting in the exposure of anti-Fas antibody to bind the Fas receptor and triggering the apoptosis of tumor cells. Results of Western blot confirmed that these NPs could mimic the function of activated cytotoxic lymphocyte (CTL) to activate the Fas–FasL apoptosis pathway of tumor cells. With the aid of CPT payload, these anti-Fas antibody conjugated NPs achieved a high tumor inhibition in the B16 allograft tumor animal model. The design of these NPs provides a method for delivering cytotoxic ligand to targeting tissue, which may be valuable in cancer therapy.
中文翻译:

抗Fas抗体偶联的纳米颗粒通过激活Fas-FasL细胞凋亡途径增强喜树碱的抗肿瘤作用
新兴证据表明,引入Fas配体(FasL)可以增强Fas依赖性细胞凋亡并诱导针对肿瘤的持久免疫应答。但是,选择性触发肿瘤细胞凋亡同时保留正常细胞仍然是应用基于FasL的治疗策略所面临的巨大挑战。本文中,制备了具有夹心结构的智能纳米颗粒(NP)。这些NP由基质金属蛋白酶(MMP)可切割的PEG外层,抗Fas抗体中间层和喜树碱(CPT)加载的内芯组成。它们可以通过增强的通透性和保留(EPR)效应积聚在肿瘤部位。可移动的PEG层可保护细胞毒性抗Fas抗体免于与正常组织的过早接触,从而避免在到达肿瘤部位之前产生意想不到的致命副作用。由于肿瘤组织内部肿瘤细胞表达的高水平的MMP,这些NP将脱落其PEG层,从而导致抗Fas抗体与Fas受体结合并触发肿瘤细胞的凋亡。Western印迹结果证实,这些NP可以模拟活化的细胞毒性淋巴细胞(CTL)激活肿瘤细胞Fas–FasL凋亡途径的功能。借助CPT有效载荷,这些抗Fas抗体偶联的NP在B16同种异体移植肿瘤动物模型中实现了高度的肿瘤抑制。这些NP的设计提供了将细胞毒性配体递送至靶向组织的方法,这在癌症治疗中可能是有价值的。导致抗Fas抗体与Fas受体结合并触发肿瘤细胞凋亡。Western印迹结果证实,这些NP可以模拟活化的细胞毒性淋巴细胞(CTL)激活肿瘤细胞Fas–FasL凋亡途径的功能。借助CPT有效载荷,这些抗Fas抗体偶联的NP在B16同种异体移植肿瘤动物模型中实现了高度的肿瘤抑制。这些NP的设计提供了将细胞毒性配体递送至靶向组织的方法,这在癌症治疗中可能是有价值的。导致抗Fas抗体与Fas受体结合并触发肿瘤细胞凋亡。Western印迹结果证实,这些NP可以模拟活化的细胞毒性淋巴细胞(CTL)激活肿瘤细胞Fas–FasL凋亡途径的功能。借助CPT有效载荷,这些抗Fas抗体偶联的NP在B16同种异体移植肿瘤动物模型中实现了高度的肿瘤抑制。这些NP的设计提供了将细胞毒性配体递送至靶向组织的方法,这在癌症治疗中可能是有价值的。这些抗Fas抗体偶联的NP在B16同种异体移植肿瘤动物模型中实现了高度的肿瘤抑制作用。这些NP的设计提供了将细胞毒性配体递送至靶向组织的方法,这在癌症治疗中可能是有价值的。这些抗Fas抗体偶联的NP在B16同种异体移植肿瘤动物模型中实现了高度的肿瘤抑制作用。这些NP的设计提供了将细胞毒性配体递送至靶向组织的方法,这在癌症治疗中可能是有价值的。
更新日期:2016-10-26
中文翻译:

抗Fas抗体偶联的纳米颗粒通过激活Fas-FasL细胞凋亡途径增强喜树碱的抗肿瘤作用
新兴证据表明,引入Fas配体(FasL)可以增强Fas依赖性细胞凋亡并诱导针对肿瘤的持久免疫应答。但是,选择性触发肿瘤细胞凋亡同时保留正常细胞仍然是应用基于FasL的治疗策略所面临的巨大挑战。本文中,制备了具有夹心结构的智能纳米颗粒(NP)。这些NP由基质金属蛋白酶(MMP)可切割的PEG外层,抗Fas抗体中间层和喜树碱(CPT)加载的内芯组成。它们可以通过增强的通透性和保留(EPR)效应积聚在肿瘤部位。可移动的PEG层可保护细胞毒性抗Fas抗体免于与正常组织的过早接触,从而避免在到达肿瘤部位之前产生意想不到的致命副作用。由于肿瘤组织内部肿瘤细胞表达的高水平的MMP,这些NP将脱落其PEG层,从而导致抗Fas抗体与Fas受体结合并触发肿瘤细胞的凋亡。Western印迹结果证实,这些NP可以模拟活化的细胞毒性淋巴细胞(CTL)激活肿瘤细胞Fas–FasL凋亡途径的功能。借助CPT有效载荷,这些抗Fas抗体偶联的NP在B16同种异体移植肿瘤动物模型中实现了高度的肿瘤抑制。这些NP的设计提供了将细胞毒性配体递送至靶向组织的方法,这在癌症治疗中可能是有价值的。导致抗Fas抗体与Fas受体结合并触发肿瘤细胞凋亡。Western印迹结果证实,这些NP可以模拟活化的细胞毒性淋巴细胞(CTL)激活肿瘤细胞Fas–FasL凋亡途径的功能。借助CPT有效载荷,这些抗Fas抗体偶联的NP在B16同种异体移植肿瘤动物模型中实现了高度的肿瘤抑制。这些NP的设计提供了将细胞毒性配体递送至靶向组织的方法,这在癌症治疗中可能是有价值的。导致抗Fas抗体与Fas受体结合并触发肿瘤细胞凋亡。Western印迹结果证实,这些NP可以模拟活化的细胞毒性淋巴细胞(CTL)激活肿瘤细胞Fas–FasL凋亡途径的功能。借助CPT有效载荷,这些抗Fas抗体偶联的NP在B16同种异体移植肿瘤动物模型中实现了高度的肿瘤抑制。这些NP的设计提供了将细胞毒性配体递送至靶向组织的方法,这在癌症治疗中可能是有价值的。这些抗Fas抗体偶联的NP在B16同种异体移植肿瘤动物模型中实现了高度的肿瘤抑制作用。这些NP的设计提供了将细胞毒性配体递送至靶向组织的方法,这在癌症治疗中可能是有价值的。这些抗Fas抗体偶联的NP在B16同种异体移植肿瘤动物模型中实现了高度的肿瘤抑制作用。这些NP的设计提供了将细胞毒性配体递送至靶向组织的方法,这在癌症治疗中可能是有价值的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号