Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Plasma-driven non-oxidative coupling of methane to ethylene and hydrogen at mild temperature over CuxO/CeO2 catalyst
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-19 , DOI: 10.1016/j.jcat.2024.115810 Rui Liu, Shangkun Li, Qian Chen, Dongxing Li, Jiasong Zhao, Chuang Li, Xiaoxia Gao, Wenping Zhao, Li Wang, Chong Peng, Annemie Bogaerts, Hongchen Guo, Yanhui Yi
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-10-19 , DOI: 10.1016/j.jcat.2024.115810 Rui Liu, Shangkun Li, Qian Chen, Dongxing Li, Jiasong Zhao, Chuang Li, Xiaoxia Gao, Wenping Zhao, Li Wang, Chong Peng, Annemie Bogaerts, Hongchen Guo, Yanhui Yi
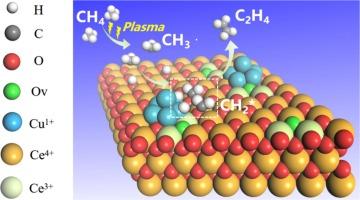
|
We report one-step non-oxidative coupling of methane (CH4 ) to ethylene (C2 H4 ) at atmospheric pressure and mild temperature (ca. 180–190 °C), by a combination of non-thermal plasma and a CuOx /CeO2 catalyst. The C2 H4 selectivity gradually increases during an induction period. The corresponding spent catalysts at different stages were systematically characterized to disclose the evolution of the CuOx /CeO2 catalyst. During the induction period, the CuO/CeO2 catalyst was partially reduced to generate Cu+ , Ce3+ and Ov species, which accompany the formation of Cu+ -Ov -Ce3+ sites, as proven by XRD, HRTEM, XPS, Raman, EPR and H2 -TPR. In addition, the C2 H4 selectivity is proportional to the fraction of Cu+ , Ce3+ , Ov and Cu-O-Ce species, which indicates that Cu+ -Ov -Ce3+ is the active site for non-oxidative coupling of CH4 to C2 H4 . Furthermore, in-situ FTIR results indicate that the Cu+ -Ov -Ce3+ interface sites can promote dehydrogenation of CH3 * (from CH4 plasma) to produce CH2 * on the catalyst surface, which is the basic reason why CuOx /CeO2 acts as a catalyst in speeding up the non-oxidative coupling of CH4 for C2 H4 production.
中文翻译:

CuxO/CeO2 催化剂在温下甲烷与乙烯和氢气的等离子体驱动非氧化耦合
我们报道了在大气压和温和温度(约 180-190 °C)下,通过非热等离子体和 CuOx/CeO2 催化剂的组合,甲烷 (CH4) 与乙烯 (C2H4) 的一步非氧化偶联。C2H4 选择性在诱导期间逐渐增加。系统表征了不同阶段的相应废催化剂,以揭示 CuOx/CeO2 催化剂的演变。在诱导期间,CuO/CeO2 催化剂被部分还原以生成 Cu+、Ce3+ 和 Ov 物种,这些物质伴随着 Cu+-Ov-Ce3+ 位点的形成,正如 XRD、HRTEM、XPS、Raman、EPR 和 H2-TPR 所证明的那样。此外,C2H4 选择性与 Cu+、Ce3+、Ov 和 Cu-O-Ce 种类的分数成正比,这表明 Cu+-Ov-Ce3+ 是 CH4 与 C2H4 非氧化偶联的活性位点。此外,原位 FTIR 结果表明,Cu+-Ov-Ce3+ 界面位点可以促进 CH3*(来自 CH4 等离子体)的脱氢,从而在催化剂表面产生 CH2*,这就是 CuOx/CeO2 作为催化剂加速 CH4 非氧化偶联以产生 C2H4 的基本原因。
更新日期:2024-10-19
中文翻译:

CuxO/CeO2 催化剂在温下甲烷与乙烯和氢气的等离子体驱动非氧化耦合
我们报道了在大气压和温和温度(约 180-190 °C)下,通过非热等离子体和 CuOx/CeO2 催化剂的组合,甲烷 (CH4) 与乙烯 (C2H4) 的一步非氧化偶联。C2H4 选择性在诱导期间逐渐增加。系统表征了不同阶段的相应废催化剂,以揭示 CuOx/CeO2 催化剂的演变。在诱导期间,CuO/CeO2 催化剂被部分还原以生成 Cu+、Ce3+ 和 Ov 物种,这些物质伴随着 Cu+-Ov-Ce3+ 位点的形成,正如 XRD、HRTEM、XPS、Raman、EPR 和 H2-TPR 所证明的那样。此外,C2H4 选择性与 Cu+、Ce3+、Ov 和 Cu-O-Ce 种类的分数成正比,这表明 Cu+-Ov-Ce3+ 是 CH4 与 C2H4 非氧化偶联的活性位点。此外,原位 FTIR 结果表明,Cu+-Ov-Ce3+ 界面位点可以促进 CH3*(来自 CH4 等离子体)的脱氢,从而在催化剂表面产生 CH2*,这就是 CuOx/CeO2 作为催化剂加速 CH4 非氧化偶联以产生 C2H4 的基本原因。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号