当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adjustment of the main biosynthesis modules to enhance the production of l‐homoserine in Escherichia coli W3110
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-10-19 , DOI: 10.1002/bit.28861 Kun Niu, Rui Zheng, Miao Zhang, Mao‐Qin Chen, Yi‐Ming Kong, Zhi‐Qiang Liu, Yu‐Guo Zheng
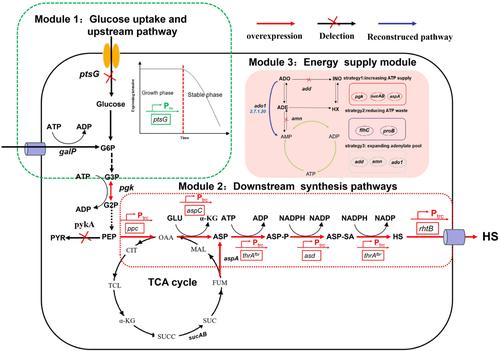
l ‐homoserine is an important platform compound of many valuable products. Construction of microbial cell factory for l ‐homoserine production from glucose has attracted a great deal of attention. In this study, l ‐homoserine biosynthesis pathway was divided into three modules, the glucose uptake and upstream pathway, the downstream pathway, and the energy supply module. Metabolomics of the chassis strain HS indicated that the supply of ATP was inadequate, therefore, the energy supply module was firstly modified. By balancing the ATP supply module, the l ‐homoserine production increased by 66% to 12.55 g/L. Further, the results indicated that the upstream pathway was blocked, and increasing the culture temperature to 37°C could solve this problem and the l ‐homoserine production reached 21.38 g/L. Then, the downstream synthesis pathways were further strengthened to balance the fluxes, and the l ‐homoserine production reached the highest reported level of 32.55 g/L in shake flasks. Finally, fed‐batch fermentation in a 5‐L bioreactor was conducted, and l ‐homoserine production could reach to 119.96 g/L after 92 h cultivation, with the yield of 0.41 g/g glucose and productivity of 1.31 g/L/h. The study provides a well research foundation for l ‐homoserine production by microbial fermentation with the capacity for industrial application.
中文翻译:

调整主要生物合成模块以提高大肠杆菌 W3110 中 l-高丝氨酸的产生
L-高丝氨酸是许多有价值的产品的重要平台化合物。用于从葡萄糖生产 l-高丝氨酸的微生物细胞工厂的建设引起了广泛关注。在本研究中,l-高丝氨酸生物合成途径分为葡萄糖摄取和上游途径、下游途径和能量供应模块三个模块。底盘应变 HS 的代谢组学表明,ATP 的供应不足,因此,首先对供能模块进行了修改。通过平衡 ATP 供应模块,l-高丝氨酸产量增加了 66%,达到 12.55 g/L。此外,结果表明上游途径受阻,将培养温度提高到 37°C 可以解决这个问题,l-高丝氨酸产量达到 21.38 g/L。然后,进一步加强下游合成途径以平衡通量,摇瓶中 l-高丝氨酸产量达到最高报道水平 32.55 g/L。最后,在 5 L 生物反应器中进行补料分批发酵,培养 92 h 后 l-高丝氨酸产量可达 119.96 g/L/L,葡萄糖产量为 0.41 g/g,生产率为 1.31 g/L/h。该研究为微生物发酵生产 l-高丝氨酸提供了良好的研究基础,具有工业应用能力。
更新日期:2024-10-19
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-10-19 , DOI: 10.1002/bit.28861 Kun Niu, Rui Zheng, Miao Zhang, Mao‐Qin Chen, Yi‐Ming Kong, Zhi‐Qiang Liu, Yu‐Guo Zheng
|
中文翻译:

调整主要生物合成模块以提高大肠杆菌 W3110 中 l-高丝氨酸的产生
L-高丝氨酸是许多有价值的产品的重要平台化合物。用于从葡萄糖生产 l-高丝氨酸的微生物细胞工厂的建设引起了广泛关注。在本研究中,l-高丝氨酸生物合成途径分为葡萄糖摄取和上游途径、下游途径和能量供应模块三个模块。底盘应变 HS 的代谢组学表明,ATP 的供应不足,因此,首先对供能模块进行了修改。通过平衡 ATP 供应模块,l-高丝氨酸产量增加了 66%,达到 12.55 g/L。此外,结果表明上游途径受阻,将培养温度提高到 37°C 可以解决这个问题,l-高丝氨酸产量达到 21.38 g/L。然后,进一步加强下游合成途径以平衡通量,摇瓶中 l-高丝氨酸产量达到最高报道水平 32.55 g/L。最后,在 5 L 生物反应器中进行补料分批发酵,培养 92 h 后 l-高丝氨酸产量可达 119.96 g/L/L,葡萄糖产量为 0.41 g/g,生产率为 1.31 g/L/h。该研究为微生物发酵生产 l-高丝氨酸提供了良好的研究基础,具有工业应用能力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号