Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ATTRv-V30M amyloid fibrils from heart and nerves exhibit structural homogeneity
Structure ( IF 4.4 ) Pub Date : 2024-10-17 , DOI: 10.1016/j.str.2024.09.021 Binh An Nguyen, Shumaila Afrin, Anna Yakubovska, Virender Singh, Rose Pedretti, Parker Bassett, Maja Pekala, Jaime Vaquer Alicea, Peter Kunach, Lanie Wang, Andrew Lemoff, Barbara Kluve-Beckerman, Lorena Saelices
Structure ( IF 4.4 ) Pub Date : 2024-10-17 , DOI: 10.1016/j.str.2024.09.021 Binh An Nguyen, Shumaila Afrin, Anna Yakubovska, Virender Singh, Rose Pedretti, Parker Bassett, Maja Pekala, Jaime Vaquer Alicea, Peter Kunach, Lanie Wang, Andrew Lemoff, Barbara Kluve-Beckerman, Lorena Saelices
|
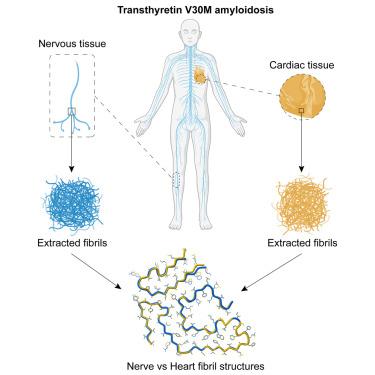
Amyloidogenic transthyretin (ATTR) amyloidosis is a systemic disease characterized by the deposition of amyloid fibrils made of transthyretin. Transthyretin is primarily produced in tetrameric form by the liver, but also by retinal epithelium and choroid plexus. The deposition of these fibrils in the myocardium and peripheral nerves causes cardiomyopathies and neuropathies, respectively. Using cryoelectron microscopy (cryo-EM), we investigated fibrils extracted from cardiac and nerve tissues of an ATTRv-V30M patient. We found consistent fibril structures from both tissues, similar to cardiac fibrils previously described, but different from vitreous humor fibrils of the same genotype. Our findings, along with previous ATTR fibrils structural studies, suggest a uniform fibrillar architecture across different tissues when transthyretin originates from the liver. This study advances our understanding of how deposition and production sites influence fibril structure in ATTRv-V30M amyloidosis.
中文翻译:

来自心脏和神经的 ATTRv-V30M 淀粉样蛋白原纤维表现出结构均匀性
淀粉样变性转甲状腺素蛋白 (ATTR) 淀粉样变性是一种全身性疾病,其特征是由转甲状腺素运载蛋白制成的淀粉样原纤维沉积。转甲状腺素蛋白主要由肝脏以四聚体形式产生,但也由视网膜上皮和脉络丛产生。这些原纤维在心肌和周围神经中的沉积分别导致心肌病和神经病。使用冷冻电子显微镜 (cryo-EM),我们研究了从 ATTRv-V30M 患者的心脏和神经组织中提取的原纤维。我们从两个组织中发现了一致的原纤维结构,类似于先前描述的心脏原纤维,但与相同基因型的玻璃体原纤维不同。我们的研究结果以及之前的 ATTR 原纤维结构研究表明,当转甲状腺素蛋白起源于肝脏时,不同组织具有均匀的原纤维结构。这项研究促进了我们对沉积和生产位点如何影响 ATTRv-V30M 淀粉样变性中原纤维结构的理解。
更新日期:2024-10-17
中文翻译:

来自心脏和神经的 ATTRv-V30M 淀粉样蛋白原纤维表现出结构均匀性
淀粉样变性转甲状腺素蛋白 (ATTR) 淀粉样变性是一种全身性疾病,其特征是由转甲状腺素运载蛋白制成的淀粉样原纤维沉积。转甲状腺素蛋白主要由肝脏以四聚体形式产生,但也由视网膜上皮和脉络丛产生。这些原纤维在心肌和周围神经中的沉积分别导致心肌病和神经病。使用冷冻电子显微镜 (cryo-EM),我们研究了从 ATTRv-V30M 患者的心脏和神经组织中提取的原纤维。我们从两个组织中发现了一致的原纤维结构,类似于先前描述的心脏原纤维,但与相同基因型的玻璃体原纤维不同。我们的研究结果以及之前的 ATTR 原纤维结构研究表明,当转甲状腺素蛋白起源于肝脏时,不同组织具有均匀的原纤维结构。这项研究促进了我们对沉积和生产位点如何影响 ATTRv-V30M 淀粉样变性中原纤维结构的理解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号