当前位置:
X-MOL 学术
›
Am. J. Hematol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Avatrombopag in immune thrombocytopenia: A real-world study of the Spanish ITP Group (GEPTI)
American Journal of Hematology ( IF 10.1 ) Pub Date : 2024-10-12 , DOI: 10.1002/ajh.27498 Cristina Pascual-Izquierdo, Blanca Sánchez-González, Mariana-Isabel Canaro-Hirnyk, Gloria García-Donas, María Menor-Gómez, Juan-José Gil-Fernández, Silvia Monsalvo-Saornil, Almudena de-Laiglesia, María-Teresa Álvarez-Román, Isidro Jarque-Ramos, María-José Llácer, Begoña Pedrote-Amador, Denis Zafra-Torres, Isabel Caparrós-Miranda, Ariana Ortúzar-Pasalodos, Nuria Revilla-Calvo, José-María Bastida, Esther Chica-Gullón, Montserrat Alvarellos, Reyes Jiménez-Bárcenas, Silvia Bernat, Daniel Martínez-Carballeira, Sunil Lakhwani, Elsa López-Ansoar, María-Esperanza Moreno-Beltrán, Álvaro Lorenzo-Vizcaya, María-Aránzazu Aguirre, Maialen Lasa-Eguialde, Marta Canet, Isabel-Teresa González-Gascón-y-Marín, Gonzalo Caballero-Navarro, Amalia Cuesta, Marta Díaz-López, Teresa Arquero, Marta Moreno-Carbonell, María-Eva Mingot-Castellano
American Journal of Hematology ( IF 10.1 ) Pub Date : 2024-10-12 , DOI: 10.1002/ajh.27498 Cristina Pascual-Izquierdo, Blanca Sánchez-González, Mariana-Isabel Canaro-Hirnyk, Gloria García-Donas, María Menor-Gómez, Juan-José Gil-Fernández, Silvia Monsalvo-Saornil, Almudena de-Laiglesia, María-Teresa Álvarez-Román, Isidro Jarque-Ramos, María-José Llácer, Begoña Pedrote-Amador, Denis Zafra-Torres, Isabel Caparrós-Miranda, Ariana Ortúzar-Pasalodos, Nuria Revilla-Calvo, José-María Bastida, Esther Chica-Gullón, Montserrat Alvarellos, Reyes Jiménez-Bárcenas, Silvia Bernat, Daniel Martínez-Carballeira, Sunil Lakhwani, Elsa López-Ansoar, María-Esperanza Moreno-Beltrán, Álvaro Lorenzo-Vizcaya, María-Aránzazu Aguirre, Maialen Lasa-Eguialde, Marta Canet, Isabel-Teresa González-Gascón-y-Marín, Gonzalo Caballero-Navarro, Amalia Cuesta, Marta Díaz-López, Teresa Arquero, Marta Moreno-Carbonell, María-Eva Mingot-Castellano
|
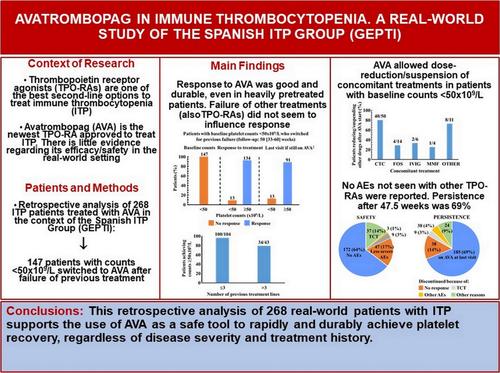
Avatrombopag is the newest thrombopoietin receptor agonist (TPO-RA) approved to treat immune thrombocytopenia (ITP). Real-world evidence regarding effectiveness/safety is limited. The Spanish ITP Group (GEPTI) performed a retrospective study with patients starting avatrombopag for the first time. A total of 268 ITP patients were recruited. The median (interquartile range [IQR]) follow-up time was 47.5 (30.4–58.9) weeks. Among the 193 patients with baseline platelet counts <50 × 109/L, 174 (90.1%) of them achieved response (PC ≥50 × 109/L), and 113 (87.6%) of the 129 who persisted on avatrombopag at last visit had platelet levels above such threshold. Results were similar when only those patients switching to avatrombopag due to previous treatment failure were considered (n = 104). Patients reached response in 13 (7–21) days. Among patients with baseline levels ≥50 × 109/L, 73/75 (97.3%) reported response, which was maintained by 53 (94.6%) of the 56 who continued on avatrombopag at the end of the study. Loss-of-response was always <10%. ITP duration did not influence response. Approximately 79% (34/43) of heavily pretreated (≥4 lines) patients with baseline platelet counts <50 × 109/L switching after previous failure achieved PC ≥50 × 109/L. Previous use of eltrombopag and/or romiplostim did not influence response, regardless of whether previous TPO-RA(s) succeeded or failed. Avatrombopag allowed dose-reduction/suspension of corticosteroids in 40/50 (80.0%) patients with baseline platelet counts <50 × 109/L. Overall, 40/268 (14.9%) thrombocytosis and 12/268 (4.5%) thromboembolic events were reported. Our real-world cohort supports the use of avatrombopag to manage ITP, regardless of disease severity and treatment history.
中文翻译:

Avatrombopag 治疗免疫性血小板减少症:西班牙 ITP 组 (GEPTI) 的真实世界研究
Avatrombopag 是最新的血小板生成素受体激动剂 (TPO-RA),被批准用于治疗免疫性血小板减少症 (ITP)。关于有效性/安全性的真实世界证据有限。西班牙 ITP 小组 (GEPTI) 对首次开始使用 avatrombopag 的患者进行了一项回顾性研究。共招募了 268 例 ITP 患者。中位 (四分位距 [IQR])随访时间为 47.5 (30.4-58.9) 周。在 193 例基线血小板计数 <50 × 109/L 的患者中,174 例 (90.1%) 达到反应 (PC ≥50 × 109/L),最后一次就诊时坚持服用阿伐曲波帕的 129 例患者中有 113 例 (87.6%) 血小板水平高于该阈值。当仅考虑那些因既往治疗失败而改用阿伐曲波帕的患者时,结果相似 (n = 104)。患者在 13 (7-21) 天内达到反应。在基线水平 ≥50 × 109/L 的患者中,73/75 (97.3%) 报告了反应,在研究结束时继续服用阿伐曲波帕的 56 例患者中,有 53 例 (94.6%) 维持了反应。响应丢失始终为 <10%。ITP 持续时间不影响反应。大约 79% (34/43) 的经过大量预处理 (≥4 线) 的患者基线血小板计数 <50 × 109/L 在先前失败后达到 PC ≥50 × 109/L。既往使用艾曲波帕和/或罗米司亭不会影响反应,无论既往 TPO-RA 是成功还是失败。Avatrombopag 允许基线血小板计数 <50 × 109/L 的 40/50 (80.0%) 患者减少剂量/暂停皮质类固醇。总体而言,报告了 40/268 (14.9%) 血小板增多和 12/268 (4.5%) 血栓栓塞事件。 我们的真实世界队列支持使用 avatrombopag 来管理 ITP,无论疾病严重程度和治疗史如何。
更新日期:2024-10-12
中文翻译:

Avatrombopag 治疗免疫性血小板减少症:西班牙 ITP 组 (GEPTI) 的真实世界研究
Avatrombopag 是最新的血小板生成素受体激动剂 (TPO-RA),被批准用于治疗免疫性血小板减少症 (ITP)。关于有效性/安全性的真实世界证据有限。西班牙 ITP 小组 (GEPTI) 对首次开始使用 avatrombopag 的患者进行了一项回顾性研究。共招募了 268 例 ITP 患者。中位 (四分位距 [IQR])随访时间为 47.5 (30.4-58.9) 周。在 193 例基线血小板计数 <50 × 109/L 的患者中,174 例 (90.1%) 达到反应 (PC ≥50 × 109/L),最后一次就诊时坚持服用阿伐曲波帕的 129 例患者中有 113 例 (87.6%) 血小板水平高于该阈值。当仅考虑那些因既往治疗失败而改用阿伐曲波帕的患者时,结果相似 (n = 104)。患者在 13 (7-21) 天内达到反应。在基线水平 ≥50 × 109/L 的患者中,73/75 (97.3%) 报告了反应,在研究结束时继续服用阿伐曲波帕的 56 例患者中,有 53 例 (94.6%) 维持了反应。响应丢失始终为 <10%。ITP 持续时间不影响反应。大约 79% (34/43) 的经过大量预处理 (≥4 线) 的患者基线血小板计数 <50 × 109/L 在先前失败后达到 PC ≥50 × 109/L。既往使用艾曲波帕和/或罗米司亭不会影响反应,无论既往 TPO-RA 是成功还是失败。Avatrombopag 允许基线血小板计数 <50 × 109/L 的 40/50 (80.0%) 患者减少剂量/暂停皮质类固醇。总体而言,报告了 40/268 (14.9%) 血小板增多和 12/268 (4.5%) 血栓栓塞事件。 我们的真实世界队列支持使用 avatrombopag 来管理 ITP,无论疾病严重程度和治疗史如何。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号