Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cryo-EM structures of Clostridium perfringens enterotoxin bound to its human receptor, claudin-4
Structure ( IF 4.4 ) Pub Date : 2024-10-08 , DOI: 10.1016/j.str.2024.09.015 Sewwandi S. Rathnayake, Satchal K. Erramilli, Anthony A. Kossiakoff, Alex J. Vecchio
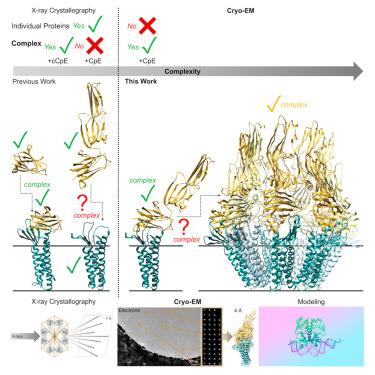
Clostridium perfringens enterotoxin (CpE) causes prevalent and deadly gastrointestinal disorders. CpE binds to receptors called claudins on the apical surfaces of small intestinal epithelium. Claudins normally regulate paracellular transport but are hijacked from doing so by CpE and are instead led to form claudin/CpE complexes. Claudin/CpE complexes are the building blocks of oligomeric β-barrel pores that penetrate the plasma membrane and induce gut cytotoxicity. Here, we present the structures of CpE in complex with its native claudin receptor in humans, claudin-4, using cryogenic electron microscopy. The structures reveal the architecture of the claudin/CpE complex, the residues used in binding, the orientation of CpE relative to the membrane, and CpE-induced changes to claudin-4. Further, structures and modeling allude to the biophysical procession from claudin/CpE complexes to cytotoxic β-barrel pores during pathogenesis. In full, this work proposes a model of claudin/CpE assembly and provides strategies to obstruct its formation to treat CpE diseases.
中文翻译:

产气荚膜梭菌肠毒素与其人类受体 claudin-4 结合的冷冻电镜结构
产气荚膜梭菌肠毒素 (CpE) 会导致普遍和致命的胃肠道疾病。CpE 与小肠上皮顶端表面称为 claudins 的受体结合。Claudin 通常调节细胞旁运输,但被 CpE 劫持,而是被引导形成 claudin/CpE 复合物。Claudin/CpE 复合物是低聚 β 桶孔的组成部分,可穿透质膜并诱导肠道细胞毒性。在这里,我们使用低温电子显微镜展示了 CpE 与其在人类中的天然 claudin 受体 claudin-4 的复合物结构。这些结构揭示了 claudin/CpE 复合物的结构、结合中使用的残基、CpE 相对于膜的方向以及 CpE 诱导的 claudin-4 变化。此外,结构和建模暗示了发病机制过程中从密蛋白/CpE 复合物到细胞毒性β桶孔的生物物理过程。总的来说,这项工作提出了一种 claudin/CpE 组装模型,并提供了阻止其形成以治疗 CpE 疾病的策略。
更新日期:2024-10-08
Structure ( IF 4.4 ) Pub Date : 2024-10-08 , DOI: 10.1016/j.str.2024.09.015 Sewwandi S. Rathnayake, Satchal K. Erramilli, Anthony A. Kossiakoff, Alex J. Vecchio
|
中文翻译:

产气荚膜梭菌肠毒素与其人类受体 claudin-4 结合的冷冻电镜结构
产气荚膜梭菌肠毒素 (CpE) 会导致普遍和致命的胃肠道疾病。CpE 与小肠上皮顶端表面称为 claudins 的受体结合。Claudin 通常调节细胞旁运输,但被 CpE 劫持,而是被引导形成 claudin/CpE 复合物。Claudin/CpE 复合物是低聚 β 桶孔的组成部分,可穿透质膜并诱导肠道细胞毒性。在这里,我们使用低温电子显微镜展示了 CpE 与其在人类中的天然 claudin 受体 claudin-4 的复合物结构。这些结构揭示了 claudin/CpE 复合物的结构、结合中使用的残基、CpE 相对于膜的方向以及 CpE 诱导的 claudin-4 变化。此外,结构和建模暗示了发病机制过程中从密蛋白/CpE 复合物到细胞毒性β桶孔的生物物理过程。总的来说,这项工作提出了一种 claudin/CpE 组装模型,并提供了阻止其形成以治疗 CpE 疾病的策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号