当前位置:
X-MOL 学术
›
Eur. J. Heart Fail.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diuretic dosing and outcomes with torsemide and furosemide following hospitalization for heart failure: The TRANSFORM‐HF trial
European Journal of Heart Failure ( IF 16.9 ) Pub Date : 2024-10-04 , DOI: 10.1002/ejhf.3458 Nina Nouhravesh, Stephen J. Greene, Robert Clare, Daniel Wojdyla, Kevin J. Anstrom, Eric Velazquez, Bertram Pitt, Robert J. Mentz, Mitchell A. Psotka
European Journal of Heart Failure ( IF 16.9 ) Pub Date : 2024-10-04 , DOI: 10.1002/ejhf.3458 Nina Nouhravesh, Stephen J. Greene, Robert Clare, Daniel Wojdyla, Kevin J. Anstrom, Eric Velazquez, Bertram Pitt, Robert J. Mentz, Mitchell A. Psotka
|
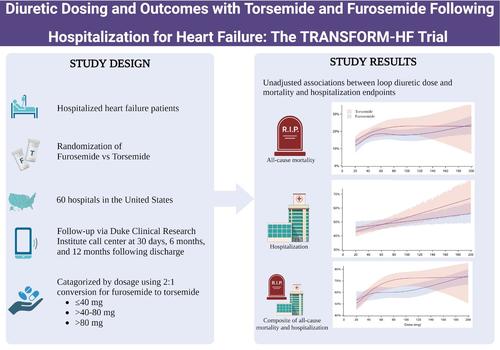
AimsThe TRANSFORM‐HF trial found no difference in clinical outcomes between torsemide versus furosemide after hospitalization for heart failure. This analysis aimed to assess the impact of diuretic dosing on the primary and secondary clinical outcomes.Methods and resultsThis post‐hoc analysis of TRANSFORM‐HF categorized patients into three groups by discharge diuretic dose: (1) ≤40 mg, (2) >40–80 mg, and (3) >80 mg of furosemide equivalents. The associations between discharge dose and 12‐month clinical events, and change in Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ‐CSS), were assessed. Overall, 2379 patients were included, aged 65 years (interquartile range 56–75), 883 (37.1%) women, and 812 (34.2%) Black. Furosemide had adjusted hazard ratios (aHR) for all‐cause mortality of 1.21 (95% confidence interval [CI] 0.91–1.59) for discharge dose group 2 and 1.40 (95% CI 1.04–1.88) for group 3, compared with group 1. For torsemide, aHRs were 1.74 (95% CI 1.32–2.30) for group 2 and 1.58 (95% CI 1.14–2.19) for group 3. No evidence of heterogeneity for the association between increased mortality and higher dose was found by loop diuretic type (p interaction = 0.17). Higher doses of furosemide and torsemide were associated with increased risk of all‐cause hospitalization and the composite of all‐cause mortality and hospitalization, without evidence of heterogeneity by loop diuretic type (p interaction > 0.2). Changes in KCCQ‐CSS from baseline at 12 months was similar across dose groups for both drugs.ConclusionFollowing hospitalization for heart failure, higher loop diuretic dosing was independently associated with worse clinical and patient‐reported outcomes. The correlation between higher loop diuretic dose and outcomes was consistent, regardless of loop diuretic used.
中文翻译:

心力衰竭住院后托拉塞米和呋塞米的利尿剂剂量和结局: TRANSFORM-HF 试验
目的TRANSFORM-HF 试验发现,因心力衰竭住院后,托拉塞米与呋塞米的临床结局没有差异。本分析旨在评估利尿剂剂量对主要和次要临床结局的影响。方法和结果这项对 TRANSFORM-HF 的事后分析按排出利尿剂剂量将患者分为三组:(1) ≤40 毫克,(2) >40-80 毫克,以及 (3) >80 毫克呋塞米当量。评估出院剂量与 12 个月临床事件之间的关联,以及堪萨斯城心肌病问卷临床总结评分 (KCCQ-CSS) 的变化。总体而言,纳入了 2379 名患者,年龄为 65 岁 (四分位距 56-75),883 名 (37.1%) 女性和 812 名 (34.2%) 黑人。与第 1 组相比,呋塞米的出院剂量组 2 全因死亡率调整后风险比 (aHR) 为 1.21 (95% 置信区间 [CI] 0.91-1.59),第 3 组为 1.40 (95% CI 1.04-1.88)。对于托拉塞米,第 2 组的 aHR 为 1.74 (95% CI 1.32-2.30),第 3 组为 1.58 (95% CI 1.14-2.19)。没有证据表明袢利尿剂类型与死亡率增加与较高剂量之间存在关联存在异质性 (pinteraction = 0.17)。较高剂量的呋塞米和托拉塞米与全因住院风险增加以及全因死亡率和住院率的复合相关,没有证据表明袢利尿剂类型存在异质性 (pinteraction > 0.2)。两种药物在 12 个月时 KCCQ-CSS 相对于基线的变化在剂量组之间相似。结论因心力衰竭住院后,较高的袢利尿剂剂量与较差的临床和患者报告结局独立相关。 无论使用何种袢利尿剂,较高袢利尿剂剂量与结局之间的相关性是一致的。
更新日期:2024-10-04
中文翻译:

心力衰竭住院后托拉塞米和呋塞米的利尿剂剂量和结局: TRANSFORM-HF 试验
目的TRANSFORM-HF 试验发现,因心力衰竭住院后,托拉塞米与呋塞米的临床结局没有差异。本分析旨在评估利尿剂剂量对主要和次要临床结局的影响。方法和结果这项对 TRANSFORM-HF 的事后分析按排出利尿剂剂量将患者分为三组:(1) ≤40 毫克,(2) >40-80 毫克,以及 (3) >80 毫克呋塞米当量。评估出院剂量与 12 个月临床事件之间的关联,以及堪萨斯城心肌病问卷临床总结评分 (KCCQ-CSS) 的变化。总体而言,纳入了 2379 名患者,年龄为 65 岁 (四分位距 56-75),883 名 (37.1%) 女性和 812 名 (34.2%) 黑人。与第 1 组相比,呋塞米的出院剂量组 2 全因死亡率调整后风险比 (aHR) 为 1.21 (95% 置信区间 [CI] 0.91-1.59),第 3 组为 1.40 (95% CI 1.04-1.88)。对于托拉塞米,第 2 组的 aHR 为 1.74 (95% CI 1.32-2.30),第 3 组为 1.58 (95% CI 1.14-2.19)。没有证据表明袢利尿剂类型与死亡率增加与较高剂量之间存在关联存在异质性 (pinteraction = 0.17)。较高剂量的呋塞米和托拉塞米与全因住院风险增加以及全因死亡率和住院率的复合相关,没有证据表明袢利尿剂类型存在异质性 (pinteraction > 0.2)。两种药物在 12 个月时 KCCQ-CSS 相对于基线的变化在剂量组之间相似。结论因心力衰竭住院后,较高的袢利尿剂剂量与较差的临床和患者报告结局独立相关。 无论使用何种袢利尿剂,较高袢利尿剂剂量与结局之间的相关性是一致的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号