Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predicting protein interactions of the kinase Lck critical to T cell modulation
Structure ( IF 4.4 ) Pub Date : 2024-10-04 , DOI: 10.1016/j.str.2024.09.010 Mu Gao, Jeffrey Skolnick
Structure ( IF 4.4 ) Pub Date : 2024-10-04 , DOI: 10.1016/j.str.2024.09.010 Mu Gao, Jeffrey Skolnick
|
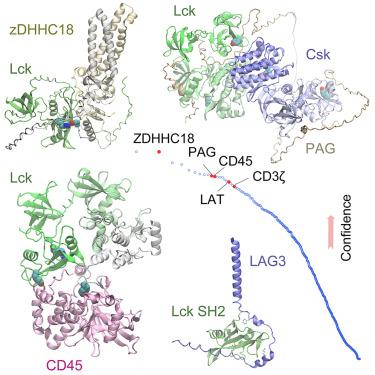
Protein-protein interactions (PPIs) play pivotal roles in directing T cell fate. One key player is the non-receptor tyrosine protein kinase Lck that helps to transduce T cell activation signals. Lck is mediated by other proteins via interactions that are inadequately understood. Here, we use the deep learning method AF2Complex to predict PPIs involving Lck, by screening it against ∼1,000 proteins implicated in immune responses, followed by extensive structural modeling for selected interactions. Remarkably, we describe how Lck may be specifically targeted by a palmitoyltransferase using a phosphotyrosine motif. We uncover “hotspot” interactions between Lck and the tyrosine phosphatase CD45, leading to a significant conformational shift of Lck for activation. Lastly, we present intriguing interactions between the phosphotyrosine-binding domain of Lck and the cytoplasmic tail of the immune checkpoint LAG3 and propose a molecular mechanism for its inhibitory role. Together, this multifaceted study provides valuable insights into T cell regulation and signaling.
中文翻译:

预测对 T 细胞调节至关重要的激酶 Lck 的蛋白质相互作用
蛋白质-蛋白质相互作用 (PPI) 在指导 T 细胞命运中起着关键作用。一个关键参与者是非受体酪氨酸蛋白激酶 Lck,它有助于转导 T 细胞活化信号。Lck 是由其他蛋白质通过尚不充分了解的相互作用介导的。在这里,我们使用深度学习方法 AF2Complex 来预测涉及 Lck 的 PPI,方法是将其与大约 1,000 种与免疫反应有关的蛋白质进行筛选,然后对选定的相互作用进行广泛的结构建模。值得注意的是,我们描述了 Lck 如何被使用磷酸酪氨酸基序的棕榈酰转移酶特异性靶向。我们发现了 Lck 和酪氨酸磷酸酶 CD45 之间的“热点”相互作用,导致 Lck 的激活发生显着构象变化。最后,我们提出了 Lck 的磷酸酪氨酸结合域与免疫检查点 LAG3 的细胞质尾部之间的有趣相互作用,并提出了其抑制作用的分子机制。总之,这项多方面的研究为 T 细胞调节和信号传导提供了有价值的见解。
更新日期:2024-10-04
中文翻译:

预测对 T 细胞调节至关重要的激酶 Lck 的蛋白质相互作用
蛋白质-蛋白质相互作用 (PPI) 在指导 T 细胞命运中起着关键作用。一个关键参与者是非受体酪氨酸蛋白激酶 Lck,它有助于转导 T 细胞活化信号。Lck 是由其他蛋白质通过尚不充分了解的相互作用介导的。在这里,我们使用深度学习方法 AF2Complex 来预测涉及 Lck 的 PPI,方法是将其与大约 1,000 种与免疫反应有关的蛋白质进行筛选,然后对选定的相互作用进行广泛的结构建模。值得注意的是,我们描述了 Lck 如何被使用磷酸酪氨酸基序的棕榈酰转移酶特异性靶向。我们发现了 Lck 和酪氨酸磷酸酶 CD45 之间的“热点”相互作用,导致 Lck 的激活发生显着构象变化。最后,我们提出了 Lck 的磷酸酪氨酸结合域与免疫检查点 LAG3 的细胞质尾部之间的有趣相互作用,并提出了其抑制作用的分子机制。总之,这项多方面的研究为 T 细胞调节和信号传导提供了有价值的见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号