当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pressure Strategy To Improve H Atomic Utilization via Optimized Decomposition Pathway in Solid Hydrazine Borane
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-09-23 , DOI: 10.1021/acs.jpclett.4c02516 Libo Sheng, Guangyu Qi, Kaixiang Jin, Ankang Chen, Xiaoli Huang, Guangtao Liu, Mi Zhou, Hongbo Wang, Yan Li, Kai Wang, Yongming Sui, Bo Zou
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-09-23 , DOI: 10.1021/acs.jpclett.4c02516 Libo Sheng, Guangyu Qi, Kaixiang Jin, Ankang Chen, Xiaoli Huang, Guangtao Liu, Mi Zhou, Hongbo Wang, Yan Li, Kai Wang, Yongming Sui, Bo Zou
|
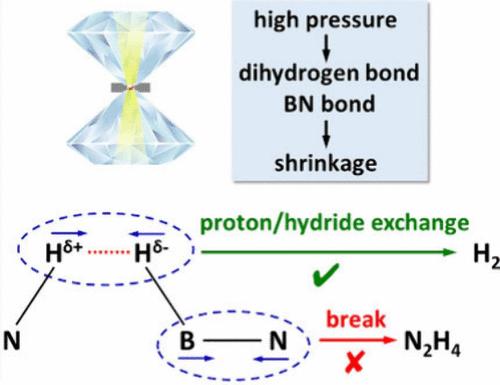
Hydrazine borane (N2H4BH3, HB), a typical B–N–H compound with very high hydrogen content (15.4 wt %), is regarded as an efficient hydrogen storage material. However, during the pyrolysis at ambient pressure, solid HB decomposes, losing ∼30 wt %, which is rationalized by the evolution of hydrazine (N2H4). Here, high pressure is introduced as an analogous catalyst role that enable to optimize the decomposition pathway of solid HB. This approach improves the H atomic utilization to over 95%. Energy-dispersive spectroscopy (EDS) analysis indicates that pressure inhibits the production of N2H4, in-situ high-pressure–high-temperature Raman and in-situ high-pressure Infrared (IR) spectra, Density functional theory (DFT) calculation, and Hirshfeld analysis reveal that this inhibition is a consequence of pressure-enhanced dihydrogen and BN bonds. The superior hydrogen release properties of HB under high pressure make it a candidate for use in the synthesis of superconductor CeH9 as a hydrogen source.
中文翻译:

通过优化固体肼硼烷分解路径提高 H 原子利用率的压力策略
肼硼烷(N 2 H 4 BH 3 ,HB)是一种典型的B-N-H化合物,具有很高的氢含量(15.4 wt%),被认为是一种高效的储氢材料。然而,在环境压力下的热解过程中,固体HB分解,损失约30wt%,这通过肼(N 2 H 4 )的放出而合理化。在这里,引入高压作为类似的催化剂作用,能够优化固体HB的分解途径。这种方法将 H 原子利用率提高到 95% 以上。能量色散光谱(EDS)分析表明压力抑制N 2 H 4的产生,原位高压高温拉曼和原位高压红外(IR)光谱,密度泛函理论(DFT)计算和赫什菲尔德分析表明,这种抑制是压力增强的二氢键和氮化硼键的结果。 HB在高压下优异的放氢性能使其成为合成超导体CeH 9作为氢源的候选材料。
更新日期:2024-09-25
中文翻译:

通过优化固体肼硼烷分解路径提高 H 原子利用率的压力策略
肼硼烷(N 2 H 4 BH 3 ,HB)是一种典型的B-N-H化合物,具有很高的氢含量(15.4 wt%),被认为是一种高效的储氢材料。然而,在环境压力下的热解过程中,固体HB分解,损失约30wt%,这通过肼(N 2 H 4 )的放出而合理化。在这里,引入高压作为类似的催化剂作用,能够优化固体HB的分解途径。这种方法将 H 原子利用率提高到 95% 以上。能量色散光谱(EDS)分析表明压力抑制N 2 H 4的产生,原位高压高温拉曼和原位高压红外(IR)光谱,密度泛函理论(DFT)计算和赫什菲尔德分析表明,这种抑制是压力增强的二氢键和氮化硼键的结果。 HB在高压下优异的放氢性能使其成为合成超导体CeH 9作为氢源的候选材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号