当前位置:
X-MOL 学术
›
Pharmacol. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Posttranslational Modifications ofα-Synuclein, Their Therapeutic Potential, and Crosstalk in Health and Neurodegenerative Diseases
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2024-11-01 , DOI: 10.1124/pharmrev.123.001111 Kambiz Hassanzadeh 1 , Jun Liu 1 , Santhosh Maddila 1 , M Maral Mouradian 2
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2024-11-01 , DOI: 10.1124/pharmrev.123.001111 Kambiz Hassanzadeh 1 , Jun Liu 1 , Santhosh Maddila 1 , M Maral Mouradian 2
Affiliation
|
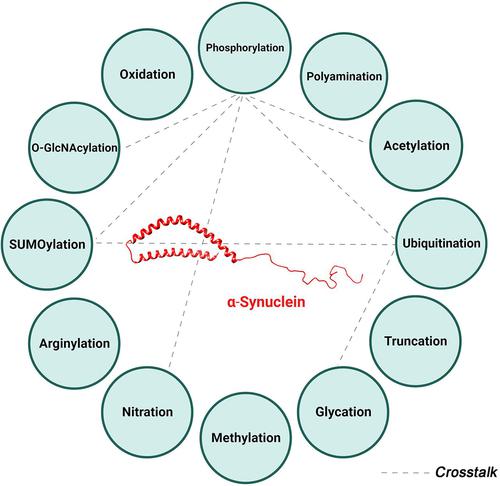
α-Synuclein (α-Syn) aggregation in Lewy bodies and Lewy neurites has emerged as a key pathogenetic feature in Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. Various factors, including posttranslational modifications (PTMs), can influence the propensity of α-Syn to misfold and aggregate. PTMs are biochemical modifications of a protein that occur during or after translation and are typically mediated by enzymes. PTMs modulate several characteristics of proteins including their structure, activity, localization, and stability. α-Syn undergoes various posttranslational modifications, including phosphorylation, ubiquitination, SUMOylation, acetylation, glycation, O-GlcNAcylation, nitration, oxidation, polyamination, arginylation, and truncation. Different PTMs of a protein can physically interact with one another or work together to influence a particular physiological or pathological feature in a process known as PTMs crosstalk. The development of detection techniques for the cooccurrence of PTMs in recent years has uncovered previously unappreciated mechanisms of their crosstalk. This has led to the emergence of evidence supporting an association between α-Syn PTMs crosstalk and synucleinopathies. In this review, we provide a comprehensive evaluation of α-Syn PTMs, their impact on misfolding and pathogenicity, the pharmacological means of targeting them, and their potential as biomarkers of disease. We also highlight the importance of the crosstalk between these PTMs in α-Syn function and aggregation. Insight into these PTMS and the complexities of their crosstalk can improve our understanding of the pathogenesis of synucleinopathies and identify novel targets of therapeutic potential.
中文翻译:

α-突触核蛋白的翻译后修饰、其治疗潜力以及健康和神经退行性疾病中的串扰
路易体和路易神经突中的 α-突触核蛋白 (α-Syn) 聚集已成为帕金森病的关键发病特征,痴呆路易体和多系统萎缩。包括翻译后修饰 (PTM) 在内的各种因素都会影响 α-Syn 错误折叠和聚集的倾向。PTM 是蛋白质的生化修饰,发生在翻译过程中或翻译之后,通常由酶介导。PTM 调节蛋白质的多种特性,包括其结构、活性、定位和稳定性。α-Syn 经历了各种翻译后修饰,包括磷酸化、泛素化、SUMO化、乙酰化、糖基化、O-GlcNAcylation、硝化、氧化、聚胺化、精氨酸化和截断。蛋白质的不同 PTM 可以相互物理相互作用或协同工作,以影响称为 PTM 串扰的过程中的特定生理或病理特征。近年来 PTM 共现检测技术的发展揭示了以前未被重视的串扰机制。这导致出现支持 α-Syn PTM 串扰与突触核蛋白病之间关联的证据。在这篇综述中,我们对 α-Syn PTMs、它们对错误折叠和致病性的影响、靶向它们的药理学手段以及它们作为疾病生物标志物的潜力进行了全面评估。我们还强调了这些 PTM 之间的串扰在 α-Syn 函数和聚集中的重要性。 深入了解这些 PTMS 及其串扰的复杂性可以提高我们对突触核蛋白病发病机制的理解,并确定具有治疗潜力的新靶点。
更新日期:2024-10-16
中文翻译:

α-突触核蛋白的翻译后修饰、其治疗潜力以及健康和神经退行性疾病中的串扰
路易体和路易神经突中的 α-突触核蛋白 (α-Syn) 聚集已成为帕金森病的关键发病特征,痴呆路易体和多系统萎缩。包括翻译后修饰 (PTM) 在内的各种因素都会影响 α-Syn 错误折叠和聚集的倾向。PTM 是蛋白质的生化修饰,发生在翻译过程中或翻译之后,通常由酶介导。PTM 调节蛋白质的多种特性,包括其结构、活性、定位和稳定性。α-Syn 经历了各种翻译后修饰,包括磷酸化、泛素化、SUMO化、乙酰化、糖基化、O-GlcNAcylation、硝化、氧化、聚胺化、精氨酸化和截断。蛋白质的不同 PTM 可以相互物理相互作用或协同工作,以影响称为 PTM 串扰的过程中的特定生理或病理特征。近年来 PTM 共现检测技术的发展揭示了以前未被重视的串扰机制。这导致出现支持 α-Syn PTM 串扰与突触核蛋白病之间关联的证据。在这篇综述中,我们对 α-Syn PTMs、它们对错误折叠和致病性的影响、靶向它们的药理学手段以及它们作为疾病生物标志物的潜力进行了全面评估。我们还强调了这些 PTM 之间的串扰在 α-Syn 函数和聚集中的重要性。 深入了解这些 PTMS 及其串扰的复杂性可以提高我们对突触核蛋白病发病机制的理解,并确定具有治疗潜力的新靶点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号