当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and intermolecular interactions in ionic liquid 1-ethyl-3-methylimidazolium bromide and its aqueous solutions investigated by vibrational spectroscopy and quantum chemical computations
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-08-14 , DOI: 10.1002/jcc.27472 Sergey A Katsyuba 1 , Stefan Grimme 2
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-08-14 , DOI: 10.1002/jcc.27472 Sergey A Katsyuba 1 , Stefan Grimme 2
Affiliation
|
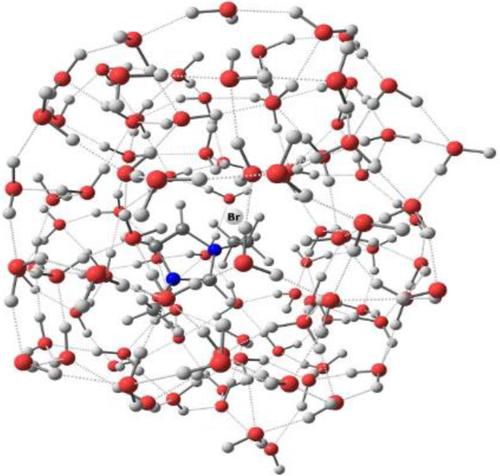
The recently developed efficient protocol to explicit quantum mechanical modeling of structure and IR spectra of liquids and solutions (S. A. Katsyuba, S. Spicher, T. P. Gerasimova, S. Grimme, J. Phys. Chem. B 2020, 124, 6664) is applied to ionic liquid (IL) 1-ethyl-3-methylimidazolium bromide (EmimBr), its C2-deuterated analog [Emim-d]Br and its aqueous solutions. It is shown that the solvation strongly modifies frequencies and IR intensities of the CH/CD stretching vibrations (νCH/νCD) of the imidazolium ring. The main vibrational spectroscopic features of the neat IL are reproduced by the simulations for a cluster (EmimBr)9, in which all three imidazolium CH moieties of the solvated cation form short contacts with three Br− anions, and another two Br− anions are located on top and bottom of imidazolium ring. Cluster models of aqueous solutions reproduce the experimental vibrational frequencies of actual solutions, provided that the Br− anion of solvated contact ion pair (CIP) is situated on top of imidazolium ring, and CH/CD moieties of the latter participate in short contacts with surrounding water molecules. Both structural and spectroscopic analysis allow to interpret the short contacts CH/CD⋯Br− and CH/CD⋯OH2 as hydrogen bonds of approximately equal strength. Enthalpies of bonding of these liquid-state H-bonds, estimated with the use of empirical correlations, amount to ca. 1.4 kcal⋅mol−1, while the analogous estimates obtained for the gas-phase charged species [Emim]2Br+ increase to 5.6 kcal⋅mol−1. It is shown that formation of solvent-shared ion pair (SIP) in aqueous solution, where the counterions of IL are separated by two water molecules H-bonded to a Br− anion, produces frequency shifts ΔνCH/CD, strongly different from the case of CIP formation. This difference can be used for IR/Raman spectroscopic differentiation of the type of solvated ion pairs of EmimBr or other related ILs.
中文翻译:

通过振动光谱和量子化学计算研究离子液体 1-乙基-3-甲基咪唑溴化物及其水溶液中的结构和分子间相互作用
最近开发的用于液体和溶液结构和红外光谱的显式量子力学建模的有效协议 (SA Katsyuba, S. Spicher, TP Gerasimova, S. Grimme, J. Phys. Chem. B 2020, 124, 6664) 应用于离子液体 (IL) 1-乙基-3-甲基咪唑溴化物 (EmimBr)、其 C2 氘代类似物 [Emim-d]Br 及其水溶液。结果表明,溶剂化强烈改变了咪唑环的 CH/CD 拉伸振动 (νCH/νCD) 的频率和 IR 强度。纯 IL 的主要振动光谱特征通过簇 (EmimBr)9 的模拟再现,其中溶剂化阳离子的所有三个咪唑鎓 CH 部分与三个 Br− 阴离子形成短接触,另外两个 Br− 阴离子位于咪唑环的顶部和底部。水溶液的聚类模型再现了实际溶液的实验振动频率,前提是溶剂化接触离子对 (CIP) 的 Br− 阴离子位于咪唑鎓环的顶部,并且后者的 CH/CD 部分参与与周围水分子的短暂接触。结构和光谱分析都可以将短接触 CH/CD⋯Br− 和 CH/CD⋯OH2 解释为强度大致相等的氢键。这些液态 H 键的键合焓,通过使用经验相关性估计,约为 1.4 kcal⋅mol-1,而为气相带电物质 [Emim]2Br+ 获得的类似估计增加到 5.6 kcal⋅mol-1。 结果表明,在水溶液中形成溶剂共享离子对 (SIP),其中 IL 的反离子被两个水分子 H 键合到 Br− 阴离子中分离,产生频移 ΔνCH/CD,这与 CIP 形成的情况截然不同。这种差异可用于 EmimBr 或其他相关 IL 的溶剂化离子对类型的红外/拉曼光谱区分。
更新日期:2024-08-14
中文翻译:

通过振动光谱和量子化学计算研究离子液体 1-乙基-3-甲基咪唑溴化物及其水溶液中的结构和分子间相互作用
最近开发的用于液体和溶液结构和红外光谱的显式量子力学建模的有效协议 (SA Katsyuba, S. Spicher, TP Gerasimova, S. Grimme, J. Phys. Chem. B 2020, 124, 6664) 应用于离子液体 (IL) 1-乙基-3-甲基咪唑溴化物 (EmimBr)、其 C2 氘代类似物 [Emim-d]Br 及其水溶液。结果表明,溶剂化强烈改变了咪唑环的 CH/CD 拉伸振动 (νCH/νCD) 的频率和 IR 强度。纯 IL 的主要振动光谱特征通过簇 (EmimBr)9 的模拟再现,其中溶剂化阳离子的所有三个咪唑鎓 CH 部分与三个 Br− 阴离子形成短接触,另外两个 Br− 阴离子位于咪唑环的顶部和底部。水溶液的聚类模型再现了实际溶液的实验振动频率,前提是溶剂化接触离子对 (CIP) 的 Br− 阴离子位于咪唑鎓环的顶部,并且后者的 CH/CD 部分参与与周围水分子的短暂接触。结构和光谱分析都可以将短接触 CH/CD⋯Br− 和 CH/CD⋯OH2 解释为强度大致相等的氢键。这些液态 H 键的键合焓,通过使用经验相关性估计,约为 1.4 kcal⋅mol-1,而为气相带电物质 [Emim]2Br+ 获得的类似估计增加到 5.6 kcal⋅mol-1。 结果表明,在水溶液中形成溶剂共享离子对 (SIP),其中 IL 的反离子被两个水分子 H 键合到 Br− 阴离子中分离,产生频移 ΔνCH/CD,这与 CIP 形成的情况截然不同。这种差异可用于 EmimBr 或其他相关 IL 的溶剂化离子对类型的红外/拉曼光谱区分。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号