当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of neoadjuvant chemoradiotherapy with or without PD-1 antibody sintilimab in pMMR locally advanced rectal cancer: A randomized clinical trial
Cancer Cell ( IF 48.8 ) Pub Date : 2024-08-01 , DOI: 10.1016/j.ccell.2024.07.004 Wei-Wei Xiao 1 , Gong Chen 2 , Yuan-Hong Gao 3 , Jun-Zhong Lin 2 , Xiao-Jun Wu 2 , Hui-Long Luo 3 , Zhen-Hai Lu 2 , Qiao-Xuan Wang 3 , Rui Sun 3 , Pei-Qiang Cai 4 , Chong-Mei Zhu 5 , Min Liu 6 , Ji-Bin Li 7 , Yi-Rui Wang 3 , Ying Jin 8 , Feng Wang 8 , Hai-Tao Luo 9 , Cai-Ling Li 9 , Zhi-Zhong Pan 2 , Rui-Hua Xu 8
Cancer Cell ( IF 48.8 ) Pub Date : 2024-08-01 , DOI: 10.1016/j.ccell.2024.07.004 Wei-Wei Xiao 1 , Gong Chen 2 , Yuan-Hong Gao 3 , Jun-Zhong Lin 2 , Xiao-Jun Wu 2 , Hui-Long Luo 3 , Zhen-Hai Lu 2 , Qiao-Xuan Wang 3 , Rui Sun 3 , Pei-Qiang Cai 4 , Chong-Mei Zhu 5 , Min Liu 6 , Ji-Bin Li 7 , Yi-Rui Wang 3 , Ying Jin 8 , Feng Wang 8 , Hai-Tao Luo 9 , Cai-Ling Li 9 , Zhi-Zhong Pan 2 , Rui-Hua Xu 8
Affiliation
|
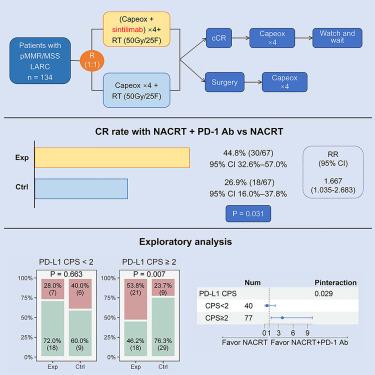
Neoadjuvant chemoradiotherapy (NACRT) was the standard treatment for patients with locally advanced rectal cancer (LARC) with proficient mismatch repair (pMMR) proteins. In this randomized phase 2 trial (ClinicalTrial.gov: NCT04304209), 134 pMMR LARC patients were randomly (1:1) assigned to receive NACRT or NACRT and the programmed cell death protein 1 (PD-1) antibody sintilimab. As the primary endpoint, the total complete response (CR) rate is 26.9% (18/67, 95% confidence interval [CI] 16.0%–37.8%) and 44.8% (30/67, 95% CI 32.6%–57.0%) in the control and experimental arm, respectively, with significant difference (p = 0.031 for chi-squared test). Response ratio is 1.667 (95% CI 1.035–2.683). Immunohistochemistry shows PD-1 ligand 1 (PD-L1) combined positive score is associated with the synergistic effect. The safety profile is similar between the arms. Adding the PD-1 antibody sintilimab to NACRT significantly increases the CR rate in pMMR LARC, with a manageable safety profile. PD-L1 positivity may help identify patients who might benefit most from the combination therapy.
中文翻译:

新辅助放化疗联合或不联合PD-1抗体信迪利单抗对pMMR局部晚期直肠癌的影响:一项随机临床试验
新辅助放化疗 (NACRT) 是具有良好错配修复 (pMMR) 蛋白的局部晚期直肠癌 (LARC) 患者的标准治疗方法。在这项随机 2 期试验 (ClinicalTrial.gov: NCT04304209) 中,134 名 pMMR LARC 患者被随机 (1:1) 分配接受 NACRT 或 NACRT 联合程序性细胞死亡蛋白 1 (PD-1) 抗体信迪利单抗治疗。作为主要终点,总完全缓解 (CR) 率为 26.9%(18/67,95% 置信区间 [CI] 16.0%–37.8%)和 44.8%(30/67,95% CI 32.6%–57.0%) )分别在对照组和实验组中具有显着差异(卡方检验 p = 0.031)。响应比为 1.667 (95% CI 1.035–2.683)。免疫组织化学显示PD-1配体1(PD-L1)联合阳性评分与协同效应相关。两臂之间的安全性相似。将 PD-1 抗体信迪利单抗添加到 NACRT 中可显着提高 pMMR LARC 的 CR 率,且安全性可控。 PD-L1 阳性可能有助于确定哪些患者可能从联合治疗中获益最多。
更新日期:2024-08-01
中文翻译:

新辅助放化疗联合或不联合PD-1抗体信迪利单抗对pMMR局部晚期直肠癌的影响:一项随机临床试验
新辅助放化疗 (NACRT) 是具有良好错配修复 (pMMR) 蛋白的局部晚期直肠癌 (LARC) 患者的标准治疗方法。在这项随机 2 期试验 (ClinicalTrial.gov: NCT04304209) 中,134 名 pMMR LARC 患者被随机 (1:1) 分配接受 NACRT 或 NACRT 联合程序性细胞死亡蛋白 1 (PD-1) 抗体信迪利单抗治疗。作为主要终点,总完全缓解 (CR) 率为 26.9%(18/67,95% 置信区间 [CI] 16.0%–37.8%)和 44.8%(30/67,95% CI 32.6%–57.0%) )分别在对照组和实验组中具有显着差异(卡方检验 p = 0.031)。响应比为 1.667 (95% CI 1.035–2.683)。免疫组织化学显示PD-1配体1(PD-L1)联合阳性评分与协同效应相关。两臂之间的安全性相似。将 PD-1 抗体信迪利单抗添加到 NACRT 中可显着提高 pMMR LARC 的 CR 率,且安全性可控。 PD-L1 阳性可能有助于确定哪些患者可能从联合治疗中获益最多。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号