Structure ( IF 4.4 ) Pub Date : 2024-07-25 , DOI: 10.1016/j.str.2024.07.001 Ieva Drulyte 1 , Rajesh Ghai 2 , Saw Yen Ow 2 , Eugene A Kapp 2 , Adam J Quek 2 , Con Panousis 2 , Michael J Wilson 2 , Andrew D Nash 2 , Matthias Pelzing 2
|
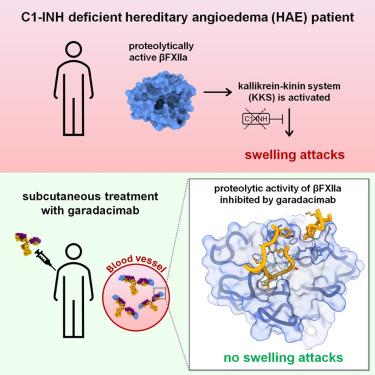
Activated FXII (FXIIa) is the principal initiator of the plasma contact system and can activate both procoagulant and proinflammatory pathways. Its activity is important in the pathophysiology of hereditary angioedema (HAE). Here, we describe a high-resolution cryoelectron microscopy (cryo-EM) structure of the beta-chain from FXIIa (βFXIIa) complexed with the Fab fragment of garadacimab. Garadacimab binds to βFXIIa through an unusually long CDR-H3 that inserts into the S1 pocket in a non-canonical way. This structural mechanism is likely the primary contributor to the inhibition of activated FXIIa proteolytic activity in HAE. Garadacimab Fab-βFXIIa structure also reveals critical determinants of high-affinity binding of garadacimab to activated FXIIa. Structural analysis with other bona fide FXIIa inhibitors, such as benzamidine and C1-INH, reveals a surprisingly similar mechanism of βFXIIa inhibition by garadacimab. In summary, the garadacimab Fab-βFXIIa structure provides crucial insights into its mechanism of action and delineates primary and auxiliary paratopes/epitopes.
中文翻译:

加拉达西单抗抑制 βFXIIa 的结构基础
活化的 FXII (FXIIa) 是血浆接触系统的主要引发剂,可以激活促凝血和促炎途径。其活性在遗传性血管性水肿 (HAE) 的病理生理学中很重要。在这里,我们描述了 FXIIa (βFXIIa) 的 β 链与加拉达西单抗 Fab 片段复合的高分辨率冷冻电子显微镜 (cryo-EM) 结构。 Garadacimab 通过异常长的 CDR-H3 与 βFXIIa 结合,该 CDR-H3 以非规范方式插入 S1 口袋。这种结构机制可能是抑制 HAE 中激活的 FXIIa 蛋白水解活性的主要因素。 Garadacimab Fab-βFXIIa 结构还揭示了 Garadacimab 与激活的 FXIIa 高亲和力结合的关键决定因素。对其他真正的 FXIIa 抑制剂(例如苯甲脒和 C1-INH)的结构分析揭示了加拉达西单抗抑制 βFXIIa 的惊人相似机制。总之,garadacimab Fab-βFXIIa 结构为其作用机制提供了重要的见解,并描绘了主要和辅助互补位/表位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号