当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cooperative Phosphine-Photoredox Catalysis Enables N–H Activation of Azoles for Intermolecular Olefin Hydroamination
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-10 , DOI: 10.1021/jacs.4c05881 Kassandra Sedillo 1 , Flora Fan 2 , Robert R Knowles 1 , Abigail G Doyle 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-07-10 , DOI: 10.1021/jacs.4c05881 Kassandra Sedillo 1 , Flora Fan 2 , Robert R Knowles 1 , Abigail G Doyle 2
Affiliation
|
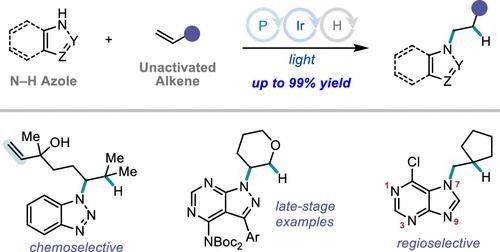
Catalytic intermolecular olefin hydroamination is an enabling synthetic strategy that offers direct and atom-economical access to a variety of nitrogen-containing compounds from abundant feedstocks. However, despite numerous advances in catalyst design and reaction development, hydroamination of N–H azoles with unactivated olefins remains an unsolved problem in synthesis. We report a dual phosphine and photoredox catalytic protocol for the hydroamination of numerous structurally diverse and medicinally relevant N–H azoles with unactivated olefins. Hydroamination proceeds with high anti-Markovnikov regioselectivity and N-site selectivity. The mild conditions and high functional group tolerance of the reaction permit the rapid construction of molecular complexity and late-stage functionalization of bioactive compounds. N–H bond activation is proposed to proceed via polar addition of the N–H azole to a phosphine radical cation, followed by P–N α-scission from a phosphoranyl radical intermediate. Reactivity and N-site selectivity are classified by azole N–H BDFE and nitrogen-centered radical spin density, respectively, which can serve as a useful predictive aid in extending the reaction to unseen azoles.
中文翻译:

膦-光氧化还原协同催化使唑类 N-H 活化,用于分子间烯烃氢胺化
催化分子间烯烃加氢胺化是一种可行的合成策略,可以以原子经济的方式从丰富的原料中直接获得各种含氮化合物。然而,尽管催化剂设计和反应开发取得了许多进展,N-H 唑与未活化烯烃的氢胺化仍然是合成中尚未解决的问题。我们报告了一种双膦和光氧化还原催化方案,用于将多种结构多样且具有药用相关性的 N-H 唑与未活化的烯烃进行加氢胺化。加氢胺化以高抗马尔可夫尼科夫区域选择性和N位选择性进行。该反应的温和条件和高官能团耐受性允许快速构建分子复杂性和生物活性化合物的后期功能化。 N-H 键活化是通过 N-H 唑与膦自由基阳离子的极性加成进行的,然后是正膦基中间体的 P-N α-断裂。反应性和N位点选择性分别按唑类 N–H BDFE 和氮中心自由基自旋密度进行分类,这可以作为将反应扩展到看不见的唑类的有用的预测辅助工具。
更新日期:2024-07-10
中文翻译:

膦-光氧化还原协同催化使唑类 N-H 活化,用于分子间烯烃氢胺化
催化分子间烯烃加氢胺化是一种可行的合成策略,可以以原子经济的方式从丰富的原料中直接获得各种含氮化合物。然而,尽管催化剂设计和反应开发取得了许多进展,N-H 唑与未活化烯烃的氢胺化仍然是合成中尚未解决的问题。我们报告了一种双膦和光氧化还原催化方案,用于将多种结构多样且具有药用相关性的 N-H 唑与未活化的烯烃进行加氢胺化。加氢胺化以高抗马尔可夫尼科夫区域选择性和N位选择性进行。该反应的温和条件和高官能团耐受性允许快速构建分子复杂性和生物活性化合物的后期功能化。 N-H 键活化是通过 N-H 唑与膦自由基阳离子的极性加成进行的,然后是正膦基中间体的 P-N α-断裂。反应性和N位点选择性分别按唑类 N–H BDFE 和氮中心自由基自旋密度进行分类,这可以作为将反应扩展到看不见的唑类的有用的预测辅助工具。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号