当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C–H activation-enabled synthesis of a piperazine-embedded azadibenzo[a,g]corannulene analogue
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-03-21 , DOI: 10.1039/d4qo00415a Lin Huang 1, 2, 3 , Mengyu Qiu 1, 2, 3 , Zhihao Chang 1, 2, 3 , Duncan L. Browne 4 , Jianhui Huang 1, 2, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-03-21 , DOI: 10.1039/d4qo00415a Lin Huang 1, 2, 3 , Mengyu Qiu 1, 2, 3 , Zhihao Chang 1, 2, 3 , Duncan L. Browne 4 , Jianhui Huang 1, 2, 3
Affiliation
|
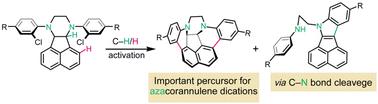
A novel piperazine-embedded azadibenzo[a,g]corannulene analogue was synthesized by a four-step bottom-up synthesis, including a nucleophilic aromatic substitution (SNAr) and a palladium-catalyzed intramolecular C–H activation arylation as key steps. This intriguing molecule represents the first example of an azacorannulene analogue bearing a piperazine ring on its polycyclic skeleton. X-ray diffraction analysis reveals a Cs-symmetric deformed bowl-shaped structure and one-dimensional columnar packing through π–π interactions with slightly offset centres, attributed to the presence of two sp3 carbons on its polycyclic core.
中文翻译:

C-H 活化合成哌嗪嵌入的氮杂二苯并[a,g]corannulene 类似物
通过四步自下而上合成,合成了一种新型哌嗪嵌入的氮杂二苯并[ a,g ]corannulene类似物,包括亲核芳香取代( SN Ar)和钯催化的分子内C-H活化芳基化作为关键步骤。这个有趣的分子代表了在其多环骨架上带有哌嗪环的氮杂环烯类似物的第一个例子。 X 射线衍射分析揭示了C s对称变形碗状结构和通过中心略微偏移的 π-π 相互作用形成的一维柱状堆积,这归因于其多环核心上存在两个 sp 3碳。
更新日期:2024-03-26
中文翻译:

C-H 活化合成哌嗪嵌入的氮杂二苯并[a,g]corannulene 类似物
通过四步自下而上合成,合成了一种新型哌嗪嵌入的氮杂二苯并[ a,g ]corannulene类似物,包括亲核芳香取代( SN Ar)和钯催化的分子内C-H活化芳基化作为关键步骤。这个有趣的分子代表了在其多环骨架上带有哌嗪环的氮杂环烯类似物的第一个例子。 X 射线衍射分析揭示了C s对称变形碗状结构和通过中心略微偏移的 π-π 相互作用形成的一维柱状堆积,这归因于其多环核心上存在两个 sp 3碳。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号