当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering of phenylalanine ammonia lyase from Rhodotorula graminis for the enhanced synthesis of unnatural l-amino acids
Tetrahedron ( IF 2.1 ) Pub Date : 2016-10-20 08:09:27 Ian Rowles, Bas Groenendaal, Baris Binay, Kirk J. Malone, Simon C. Willies, Nicholas J. Turner
Tetrahedron ( IF 2.1 ) Pub Date : 2016-10-20 08:09:27 Ian Rowles, Bas Groenendaal, Baris Binay, Kirk J. Malone, Simon C. Willies, Nicholas J. Turner
|
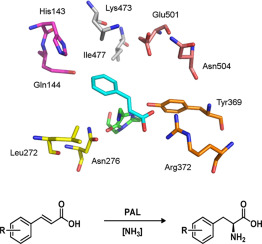
Phenylalanine ammonia lyase (PAL) catalyses the reversible non-oxidative deamination of phenylalanine to trans-cinnamic acid and ammonia. Analogues of l-phenylalanine are incorporated as pharmacophores in several peptidomimetic drug molecules and are therefore of particular interest to the fine chemical industry. PAL from Rhodotorula graminis (RgrPAL) has shown an ability to accept analogues of l-phenylalanine. Our aim was to increase enzymatic activity with directed evolution towards a specific non-natural substrate through the cloning and over-production of PAL in Escherichia coli. The identified variants of RgrPAL with significantly showed more catalytic efficient compared to the wild-type enzyme. These variants were used in a preparative scale biotransformation resulting in a 94% conversion to L-4-Br-phenylalanine (>99% ee).
中文翻译:

重金属红假单胞菌的苯丙氨酸氨裂合酶工程改造,以增强非天然L-氨基酸的合成
苯丙氨酸氨裂合酶(PAL)催化将苯丙氨酸可逆的非氧化性脱氨反应为反式肉桂酸和氨。1-苯丙氨酸的类似物作为药效团并入几种拟肽药物分子中,因此对精细化学工业特别感兴趣。Rhodotorula graminis(RgrPAL)的PAL已显示出接受1-苯丙氨酸类似物的能力。我们的目标是通过在大肠杆菌中克隆和过量生产PAL,增加针对特定非天然底物的定向进化来提高酶活性。与野生型酶相比,已鉴定的RgrPAL变体显着显示出更高的催化效率。这些变体用于制备规模的生物转化中,导致94%转化为L-4-Br-苯丙氨酸(> 99%ee)。
更新日期:2016-10-20
中文翻译:

重金属红假单胞菌的苯丙氨酸氨裂合酶工程改造,以增强非天然L-氨基酸的合成
苯丙氨酸氨裂合酶(PAL)催化将苯丙氨酸可逆的非氧化性脱氨反应为反式肉桂酸和氨。1-苯丙氨酸的类似物作为药效团并入几种拟肽药物分子中,因此对精细化学工业特别感兴趣。Rhodotorula graminis(RgrPAL)的PAL已显示出接受1-苯丙氨酸类似物的能力。我们的目标是通过在大肠杆菌中克隆和过量生产PAL,增加针对特定非天然底物的定向进化来提高酶活性。与野生型酶相比,已鉴定的RgrPAL变体显着显示出更高的催化效率。这些变体用于制备规模的生物转化中,导致94%转化为L-4-Br-苯丙氨酸(> 99%ee)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号