当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and Enantioselective Construction of Tetrazole Hemiaminal Esters and Related Prodrugs via Biocatalytic Dynamic Kinetic Resolution
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-01-22 , DOI: 10.1021/acs.joc.3c02076 Maochun Han 1 , Changming Liu 1 , Xinyu Li 1 , Jingyu Jiang 1 , Ziliang Liu 1 , Lei Hu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-01-22 , DOI: 10.1021/acs.joc.3c02076 Maochun Han 1 , Changming Liu 1 , Xinyu Li 1 , Jingyu Jiang 1 , Ziliang Liu 1 , Lei Hu 1
Affiliation
|
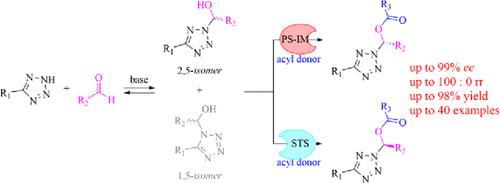
Enzyme-catalyzed dynamic kinetic resolution was applied to the one-pot regio- and enantioselective synthesis of 2,5-disubstituted tetrazole hemiaminal esters, among which 72% of the products were obtained in excellent enantiopurities (99% ees). Tunable stereoselectivity was achieved by using different types of enzymes during the synthesis of a key intermediate for a clinic drug candidate. Successful preparation of tetrazole ester prodrugs and high catalyst recyclability further demonstrated the potential practical application of this protocol.
中文翻译:

通过生物催化动态动力学拆分区域选择性和对映选择性构建四唑半缩醛胺酯及相关前药
应用酶催化动态动力学拆分方法一锅法区域选择性和对映选择性合成2,5-二取代四唑半缩醛胺酯,其中72%的产物具有优异的对映纯度(99% ee s)。在合成临床候选药物的关键中间体过程中,通过使用不同类型的酶实现了可调节的立体选择性。四唑酯前药的成功制备和高催化剂可回收性进一步证明了该方案的潜在实际应用。
更新日期:2024-01-22
中文翻译:

通过生物催化动态动力学拆分区域选择性和对映选择性构建四唑半缩醛胺酯及相关前药
应用酶催化动态动力学拆分方法一锅法区域选择性和对映选择性合成2,5-二取代四唑半缩醛胺酯,其中72%的产物具有优异的对映纯度(99% ee s)。在合成临床候选药物的关键中间体过程中,通过使用不同类型的酶实现了可调节的立体选择性。四唑酯前药的成功制备和高催化剂可回收性进一步证明了该方案的潜在实际应用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号