Chemical Engineering Science ( IF 4.1 ) Pub Date : 2023-12-12 , DOI: 10.1016/j.ces.2023.119631 Yi-Fan Xue , Jie Feng , Jin-Yao Ma , Wen-Ying Li
|
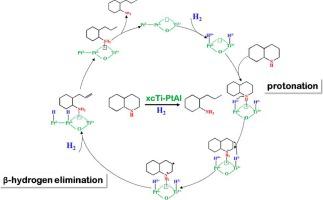
C–N bond breaking is a difficult but crucial step in the quinoline removal of coal-based liquids. Noble metal catalysts have higher C–N bond breaking activity, but their surface bond breaking mechanism is unclear. Atomic layer deposition creates more metal-oxide sites and generates different active species, which facilitates the mechanism study. For this reason, we used atomic layer deposition to deposit TiO2 on the catalyst surface and suggested a more complete C–N bond breaking mechanism in this study. The particle size and pore structure of the catalyst did not change significantly after the deposition, and the hydrogen adsorption was reduced when there were too many layers deposited and the surface Pt sites were occupied to a high degree. TiO2 is extremely susceptible to reduction to Ti3+ under hydrogen, and can change the nature of the electron density of Pt through oxygen vacancies, creating Ti3+-Ptδ− sites that are prone to protonation reactions. That means, the occurrence of these protonation reactions not only easily breaks the C–N bond, but also accelerates the rate of C–N bond cleavage due to the increased H coverage on the surface of the xcTi-PtAl catalysts. And the Ti3+-Ptδ− sites in catalyst had a stronger adsorption effect on the nitrogen-containing compounds, which in turn lowered the energy barriers of bond cleavage. It is concluded that protonation is a necessary step before the C–N bond can be broken. The subsequent steps were showed by the DFT calculations that the nitrogen-containing compounds adsorbed on the Ti3+-Ptδ− sites undergo β-H fracture after the protonation reaction, with the elimination reaction cleaving the C–N bond. The xcTi-PtAl catalysts by atomic layer deposition of Ti not only improve the C–N bond breaking performance, but also improve bond breaking stability by a factor of four compared to the Pt/Al2O3 catalysts.
中文翻译:

阐明铂基催化剂上喹啉加氢脱氮的 C-N 键断裂机制
C-N键断裂是煤基液体喹啉去除中困难但关键的一步。贵金属催化剂具有较高的C-N键断裂活性,但其表面键断裂机制尚不清楚。原子层沉积产生更多的金属氧化物位点并产生不同的活性物质,这有利于机理研究。为此,我们采用原子层沉积的方法在催化剂表面沉积TiO 2 ,并在本研究中提出了更完整的C-N键断裂机制。沉积后催化剂的粒径和孔结构没有明显变化,当沉积层数过多且表面Pt位点被高度占据时,氢吸附降低。 TiO 2 在氢气下极易还原为 Ti 3+ ,并且可以通过氧空位改变 Pt 电子密度的性质,产生 Ti 3+ - Pt δ− 位点容易发生质子化反应。这意味着,这些质子化反应的发生不仅容易破坏C-N键,而且由于xcTi-PtAl催化剂表面H覆盖率的增加,还加速了C-N键断裂的速率。催化剂中的Ti 3+ -Pt δ− 位点对含氮化合物具有更强的吸附作用,从而降低了键断裂的能垒。结论是质子化是 C-N 键断裂之前的必要步骤。随后的DFT计算表明,吸附在Ti 3+ -Pt δ− 位点上的含氮化合物在质子化反应后发生β-H断裂,发生消除反应断裂C-N键。 采用 Ti 原子层沉积的 xcTi-PtAl 催化剂不仅提高了 C-N 键断裂性能,而且与 Pt/Al 2 O 3 催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号