当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiodivergent Synthesis of Halohydrins by Engineering P450DA Monooxygenases
ACS Catalysis ( IF 13.1 ) Pub Date : 2023-11-28 , DOI: 10.1021/acscatal.3c04742
Xiao-Jian Zhou , Mao-Yao Wang , Ling Zhao , Yu-Qi He , Zhong-Qiang Wang , Jia-Jing Li , Guo-Zhong Deng , Nan-Wei Wan , Yong-Zheng Chen
ACS Catalysis ( IF 13.1 ) Pub Date : 2023-11-28 , DOI: 10.1021/acscatal.3c04742
Xiao-Jian Zhou , Mao-Yao Wang , Ling Zhao , Yu-Qi He , Zhong-Qiang Wang , Jia-Jing Li , Guo-Zhong Deng , Nan-Wei Wan , Yong-Zheng Chen
|
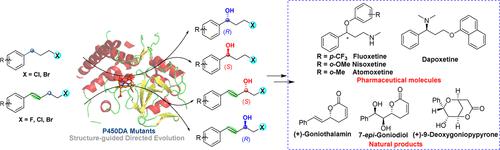
Chiral γ-halohydrins and β-haloallyl alcohols are important building blocks for the synthesis of pharmacologically active compounds. Direct enantioselective C–H bond hydroxylation of halohydrocarbons is an appealing method for the synthesis of these compounds. Herein, P450DA mutants, which could improve or reverse the enantioselectivity, were generated by structure-guided directed evolution based on the X-ray crystal structure of P450DA-M3. It catalyzed the benzylic and allylic C–H bond hydroxylation of halohydrocarbons with regio-, chemo-, and enantioselectivity and provided the desirable enantiomers of both chiral γ-halohydrins (43–94% ee) and β-haloallyl alcohols (79–96% ee), while the halogen atoms and C═C bonds in the molecule remained unreacted. This enzymatic platform represents an example of catalytic systems achieving enantiodivergent control of C–H bond hydroxylation in halohydrocarbons via protein engineering.
中文翻译:

通过工程化 P450DA 单加氧酶对映异构合成卤代醇
手性γ-卤代醇和β-卤代烯丙醇是合成药理活性化合物的重要组成部分。卤代烃的直接对映选择性 C-H 键羟基化是合成这些化合物的一种有吸引力的方法。在此,基于P450DA-M3的X射线晶体结构,通过结构引导的定向进化产生了可以提高或逆转对映选择性的P450DA突变体。它催化卤代烃的苄基和烯丙基 C-H 键羟基化,具有区域选择性、化学选择性和对映选择性,并提供了所需的手性 γ-卤代醇 (43-94% ee) 和 β-卤代烯丙醇 (79-96%) 对映体ee),而分子中的卤素原子和C=C键仍未反应。该酶平台代表了催化系统通过蛋白质工程实现卤代烃中 C-H 键羟基化对映异构控制的一个例子。
更新日期:2023-11-28
中文翻译:

通过工程化 P450DA 单加氧酶对映异构合成卤代醇
手性γ-卤代醇和β-卤代烯丙醇是合成药理活性化合物的重要组成部分。卤代烃的直接对映选择性 C-H 键羟基化是合成这些化合物的一种有吸引力的方法。在此,基于P450DA-M3的X射线晶体结构,通过结构引导的定向进化产生了可以提高或逆转对映选择性的P450DA突变体。它催化卤代烃的苄基和烯丙基 C-H 键羟基化,具有区域选择性、化学选择性和对映选择性,并提供了所需的手性 γ-卤代醇 (43-94% ee) 和 β-卤代烯丙醇 (79-96%) 对映体ee),而分子中的卤素原子和C=C键仍未反应。该酶平台代表了催化系统通过蛋白质工程实现卤代烃中 C-H 键羟基化对映异构控制的一个例子。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号