当前位置:
X-MOL 学术
›
Redox Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of sulfhydryl-containing proteins and further evaluation of the selenium-tagged redox homeostasis-regulating proteins
Redox Biology ( IF 10.7 ) Pub Date : 2023-11-27 , DOI: 10.1016/j.redox.2023.102969 Zhongyao Jiang 1 , Yue Tang 2 , Jun Lu 1 , Chang Xu 1 , Yaxin Niu 1 , Guanglu Zhang 1 , Yanmei Yang 1 , Xiufen Cheng 1 , Lili Tong 1 , Zhenzhen Chen 1 , Bo Tang 3
Redox Biology ( IF 10.7 ) Pub Date : 2023-11-27 , DOI: 10.1016/j.redox.2023.102969 Zhongyao Jiang 1 , Yue Tang 2 , Jun Lu 1 , Chang Xu 1 , Yaxin Niu 1 , Guanglu Zhang 1 , Yanmei Yang 1 , Xiufen Cheng 1 , Lili Tong 1 , Zhenzhen Chen 1 , Bo Tang 3
Affiliation
|
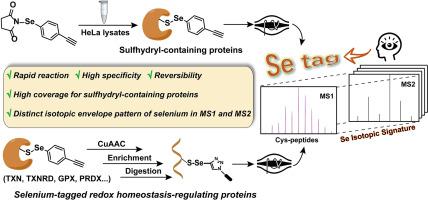
Chemoproteomic profiling of sulfhydryl-containing proteins has consistently been an attractive research hotspot. However, there remains a dearth of probes that are specifically designed for sulfhydryl-containing proteins, possessing sufficient reactivity, specificity, distinctive isotopic signature, as well as efficient labeling and evaluation capabilities for proteins implicated in the regulation of redox homeostasis. Here, the specific selenium-containing probes (Se-probes) in this work displayed high specificity and reactivity toward cysteine thiols on small molecules, peptides and purified proteins and showed very good competitive effect of proteins labeling in gel-ABPP. We identified more than 6000 candidate proteins. In TOP-ABPP, we investigated the peptide labeled by Se-probes, which revealed a distinct isotopic envelope pattern of selenium in both the primary and secondary mass spectra. This unique pattern can provide compelling evidence for identifying redox regulatory proteins and other target peptides. Furthermore, our examiation of post-translational modification (PTMs) of the cysteine site residues showed that oxidation PTMs was predominantly observed. We anticipate that Se-probes will enable broader and deeper proteome-wide profiling of sulfhydryl-containing proteins, provide an ideal tool for focusing on proteins that regulate redox homeostasis and advance the development of innovative selenium-based pharmaceuticals.
中文翻译:

含巯基蛋白质的鉴定和进一步评估硒标记的氧化还原稳态调节蛋白
含巯基蛋白质的化学蛋白质组学分析一直是一个有吸引力的研究热点。然而,仍然缺乏专门为含巯基蛋白质设计的探针,这些探针具有足够的反应性、特异性、独特的同位素特征,以及对与氧化还原稳态调节有关的蛋白质的有效标记和评估能力。在这里,这项工作中的特异性含硒探针 (Se-probes) 对小分子、肽和纯化蛋白质上的半胱氨酸硫醇表现出高特异性和反应性,并且在凝胶-ABPP 中显示出非常好的蛋白质标记竞争效应。我们鉴定了 6000 多种候选蛋白。在 TOP-ABPP 中,我们研究了用 Se 探针标记的肽,这在初级和次级质谱中都揭示了硒的独特同位素包膜模式。这种独特的模式可以为鉴定氧化还原调节蛋白和其他靶肽提供令人信服的证据。此外,我们对半胱氨酸位点残基的翻译后修饰 (PTM) 的检查表明,主要观察到氧化 PTM。我们预计 Se 探针将能够更广泛、更深入地对含巯基的蛋白质进行全蛋白质组分析,为关注调节氧化还原稳态的蛋白质和推动创新硒基药物的开发提供理想的工具。
更新日期:2023-11-27
中文翻译:

含巯基蛋白质的鉴定和进一步评估硒标记的氧化还原稳态调节蛋白
含巯基蛋白质的化学蛋白质组学分析一直是一个有吸引力的研究热点。然而,仍然缺乏专门为含巯基蛋白质设计的探针,这些探针具有足够的反应性、特异性、独特的同位素特征,以及对与氧化还原稳态调节有关的蛋白质的有效标记和评估能力。在这里,这项工作中的特异性含硒探针 (Se-probes) 对小分子、肽和纯化蛋白质上的半胱氨酸硫醇表现出高特异性和反应性,并且在凝胶-ABPP 中显示出非常好的蛋白质标记竞争效应。我们鉴定了 6000 多种候选蛋白。在 TOP-ABPP 中,我们研究了用 Se 探针标记的肽,这在初级和次级质谱中都揭示了硒的独特同位素包膜模式。这种独特的模式可以为鉴定氧化还原调节蛋白和其他靶肽提供令人信服的证据。此外,我们对半胱氨酸位点残基的翻译后修饰 (PTM) 的检查表明,主要观察到氧化 PTM。我们预计 Se 探针将能够更广泛、更深入地对含巯基的蛋白质进行全蛋白质组分析,为关注调节氧化还原稳态的蛋白质和推动创新硒基药物的开发提供理想的工具。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号