当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Pyrido-[2,1-a]isoquinoline Derivatives via Gold(I)-Catalyzed Intramolecular Cascade Reaction of Enamides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-11-23 , DOI: 10.1002/adsc.202300908 Xin-Ming Xu 1 , Sen Chen 1 , Wenzhong Li 1 , Xuesi Zhang 1 , Zhongyuan Zhao 1 , Xinyue Cao 1 , Yuhang Yao 1 , Xiangmin Wang 1 , Zu-Li Wang 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2023-11-23 , DOI: 10.1002/adsc.202300908 Xin-Ming Xu 1 , Sen Chen 1 , Wenzhong Li 1 , Xuesi Zhang 1 , Zhongyuan Zhao 1 , Xinyue Cao 1 , Yuhang Yao 1 , Xiangmin Wang 1 , Zu-Li Wang 2
Affiliation
|
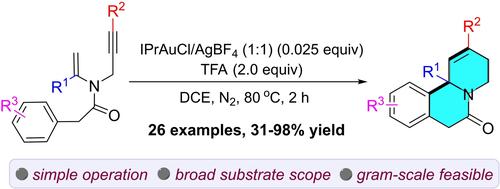
A gold(I)-catalyzed intramolecular cascade reaction of tertiary enamides has been developed, which provides a method for the preparation of diverse pyrido[2,1-a]isoquinoline derivatives with good function group tolerance. Preliminary investigation of the reaction mechanism indicate that this process comprises a nucleophilic addition of the enamide to an alkynyl group, followed by the capture of the acyliminium ion by an electron-rich aromatic component. Additionally, scale-up experiments and further transformations of pyrido-[2,1-a]isoquinolines underscore the utility of this methodology.
中文翻译:

金(I)催化烯酰胺分子内级联反应合成吡啶并-[2,1-a]异喹啉衍生物
开发了金(I)催化的叔烯酰胺分子内级联反应,为制备具有良好官能团耐受性的多种吡啶并[2,1-a]异喹啉衍生物提供了一种方法。反应机理的初步研究表明,该过程包括烯酰胺与炔基的亲核加成,然后由富电子芳香族组分捕获酰亚胺离子。此外,吡啶并-[2,1-a]异喹啉的放大实验和进一步转化强调了该方法的实用性。
更新日期:2023-11-23
中文翻译:

金(I)催化烯酰胺分子内级联反应合成吡啶并-[2,1-a]异喹啉衍生物
开发了金(I)催化的叔烯酰胺分子内级联反应,为制备具有良好官能团耐受性的多种吡啶并[2,1-a]异喹啉衍生物提供了一种方法。反应机理的初步研究表明,该过程包括烯酰胺与炔基的亲核加成,然后由富电子芳香族组分捕获酰亚胺离子。此外,吡啶并-[2,1-a]异喹啉的放大实验和进一步转化强调了该方法的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号