当前位置:
X-MOL 学术
›
J. Pharmaceut. Biomed. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analytical validation of the PROphet test for treatment decision-making guidance in metastatic non-small cell lung cancer
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.1 ) Pub Date : 2023-10-19 , DOI: 10.1016/j.jpba.2023.115803 Ben Yellin 1 , Coren Lahav 1 , Itamar Sela 1 , Galit Yahalom 1 , Shani Raveh Shoval 1 , Yehonatan Elon 1 , James Fuller 2 , Michal Harel 1
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.1 ) Pub Date : 2023-10-19 , DOI: 10.1016/j.jpba.2023.115803 Ben Yellin 1 , Coren Lahav 1 , Itamar Sela 1 , Galit Yahalom 1 , Shani Raveh Shoval 1 , Yehonatan Elon 1 , James Fuller 2 , Michal Harel 1
Affiliation
|
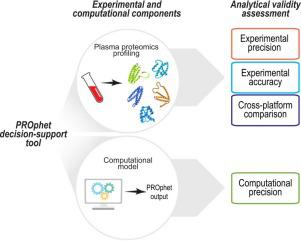
The blood proteome, consisting of thousands of proteins engaged in various biological processes, acts as a valuable source of potential biomarkers for various medical applications. PROphet is a plasma proteomics-based test that serves as a decision-support tool for non-small cell lung cancer (NSCLC) patients, combining proteomic profiling using SomaScan technology and subsequent computational algorithm. PROphet was implemented as a laboratory developed test (LDT). Under the Clinical Laboratory Improvement Amendments (CLIA) and Commission on Office Laboratory Accreditation (COLA) regulations, prior to releasing patient test results, a clinical laboratory located in the United States employing an LDT must examine its performance characteristics with regard to analytical validity. This study describes the experimental and computational analytical validity of the PROphet test, as required by CLIA/COLA regulations. Experimental precision analysis displayed a median coefficient of variation (CV) of 3.9 % and 4.7 % for intra-plate and inter-plate examination, respectively, and the median accuracy rate between sites was 88 %. Computational precision exhibited a high accuracy rate, with 93 % of samples displaying complete concordance in results. A cross-platform comparison between SomaScan and other proteomics platforms yielded a median Spearman's rank correlation coefficient of 0.51, affirming the consistency and reliability of the SomaScan platform as used under the PROphet test. Our study presents a robust framework for evaluating the analytical validity of a platform that combines an experimental assay with subsequent computational algorithms. When applied to the PROphet test, strong analytical performance of the test was demonstrated.
中文翻译:

PROphet 测试对转移性非小细胞肺癌治疗决策指导的分析验证
血液蛋白质组由参与各种生物过程的数千种蛋白质组成,是各种医学应用的潜在生物标志物的宝贵来源。 PROphet 是一种基于血浆蛋白质组学的测试,可作为非小细胞肺癌 (NSCLC) 患者的决策支持工具,结合使用 SomaScan 技术和后续计算算法进行蛋白质组学分析。 PROphet 作为实验室开发的测试 (LDT) 实施。根据临床实验室改进修正案 (CLIA) 和办公室实验室认证委员会 (COLA) 的规定,在发布患者检测结果之前,位于美国的使用 LDT 的临床实验室必须检查其分析有效性方面的性能特征。本研究按照 CLIA/COLA 法规的要求描述了 PROphet 测试的实验和计算分析有效性。实验精密度分析显示,板内和板间检查的中位变异系数(CV)分别为3.9%和4.7%,位点间的中位准确率为88%。计算精度表现出较高的准确率,93%的样本结果完全一致。 SomaScan 与其他蛋白质组学平台之间的跨平台比较得出 Spearman 等级相关系数中位数为 0.51,证实了 PROphet 测试中使用的 SomaScan 平台的一致性和可靠性。我们的研究提出了一个强大的框架,用于评估将实验测定与后续计算算法相结合的平台的分析有效性。当应用于PROphet测试时,证明了该测试强大的分析性能。
更新日期:2023-10-19
中文翻译:

PROphet 测试对转移性非小细胞肺癌治疗决策指导的分析验证
血液蛋白质组由参与各种生物过程的数千种蛋白质组成,是各种医学应用的潜在生物标志物的宝贵来源。 PROphet 是一种基于血浆蛋白质组学的测试,可作为非小细胞肺癌 (NSCLC) 患者的决策支持工具,结合使用 SomaScan 技术和后续计算算法进行蛋白质组学分析。 PROphet 作为实验室开发的测试 (LDT) 实施。根据临床实验室改进修正案 (CLIA) 和办公室实验室认证委员会 (COLA) 的规定,在发布患者检测结果之前,位于美国的使用 LDT 的临床实验室必须检查其分析有效性方面的性能特征。本研究按照 CLIA/COLA 法规的要求描述了 PROphet 测试的实验和计算分析有效性。实验精密度分析显示,板内和板间检查的中位变异系数(CV)分别为3.9%和4.7%,位点间的中位准确率为88%。计算精度表现出较高的准确率,93%的样本结果完全一致。 SomaScan 与其他蛋白质组学平台之间的跨平台比较得出 Spearman 等级相关系数中位数为 0.51,证实了 PROphet 测试中使用的 SomaScan 平台的一致性和可靠性。我们的研究提出了一个强大的框架,用于评估将实验测定与后续计算算法相结合的平台的分析有效性。当应用于PROphet测试时,证明了该测试强大的分析性能。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号