Synlett ( IF 1.7 ) Pub Date : 2023-10-18 , DOI: 10.1055/s-0042-1751508 Yoshiyuki Hari , Yasufumi Fuchi , Miho Kawaguchi , Yuta Ito
|
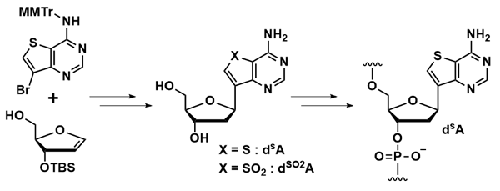
A C-nucleoside with a stable C–C glycosidic bond can be used as a building block for chemically modified oligonucleotides (ONs). In this study, two adenosine-like C-nucleosides (dSA and dSO2A) bearing thieno[3,2-d]pyrimidine rings were designed and synthesized. These analogues were synthesized via the Heck reaction, and their properties as monomer nucleosides were investigated. Both the dSA and dSO2A monomers were not recognized by adenosine deaminase (ADA). In addition, they exhibited fluorescence emissions in the UV and visible regions of dSA and dSO2A, respectively. Subsequently, dSA was converted into a phosphoramidite compound and incorporated into the ONs. The synthesized dSA-modified ONs formed a stable duplex with DNA and RNA complements comparable to natural adenosine. Furthermore, the modified ONs exhibited fluorescence emission derived from dSA.
中文翻译:

噻吩并[3,2-d]嘧啶环2′-脱氧腺苷模拟物的合成及性能
具有稳定 C-C 糖苷键的 C-核苷可用作化学修饰寡核苷酸 (ON) 的构建模块。在本研究中,设计并合成了两种带有噻吩并[3,2- d ]嘧啶环的腺苷样C核苷(dSA和dSO2A )。这些类似物是通过 Heck 反应合成的,并研究了它们作为单体核苷的性质。dSA和 dSO2A单体均不被腺苷脱氨酶 (ADA) 识别。此外,它们分别在 d SA和 d SO2 A 的紫外和可见光区域表现出荧光发射。随后,d SA转化为亚磷酰胺化合物并掺入 ON 中。合成的 dSA修饰的 ON 与 DNA 和 RNA 互补物形成稳定的双链体,与天然腺苷相当。此外,修饰后的 ON 表现出源自 d SA 的荧光发射。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号