Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-10-10 , DOI: 10.1016/j.molliq.2023.123256 Xiaobo Cai , Chaohui Yang , Li Chen , Rongrong Li , Cunbin Du
|
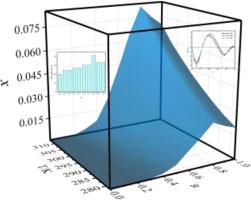
This research investigates the cosolvent effect of nirmatrelvir (treatment of COVID-19) in ethanol + water mixtures. The result shows that the maximum solubility in mole fraction is 8.313 × 10−2 at the ethanol mass fraction of 80%, which is greater than that in pure water. The solvent effect analysis indicates that the hydrogen bond acidity (HBA) of solvent has the greatest impact on the solubility of nirmatrelvir solubility. The negative preferential solvation values in water-rich regions indicate that nirmatrelvir molecules are preferentially solvated by water molecules. With the rising mass fraction of cosolvent, ethanol disrupts the ordered structure of water molecules in solution through hydrogen bonding, allowing nirmatrelvir molecules to be preferentially solvated by ethanol. Nonetheless, nirmatrelvir molecules are preferentially solvated by water again in ethanol-rich regions. Through molecular dynamics calculations (MD), it could be found that the dissolution behavior is consistent with its calculated by solvation free energy calculation. Furthermore, the tall and sharp peak of the RDF plots of O···H-O (ethanol/water) and N-H···O (ethanol/water) were attained at 1.50–2.50 Å, indicating that ethanol and water molecules form a regular and definite coordination sphere around the nirmatrelvir moiety at the distance of 1.50–2.50 Å through hydrogen bonding form. Additionally, the correlation fitting was performed for the solubility data through the Jouyban-Acree model and its derivative models. Moreover, “Apparent thermodynamic analysis” of nirmatrelvir dissolution process in aqueous ethanol mixtures including entropy, enthalpy and Gibbs free energy were completed.
中文翻译:

Nirmatrelvir(治疗COVID-19)在乙醇水溶液中的溶出机理、分子动力学模拟和热力学分析
本研究调查了 nirmatrelvir(治疗 COVID-19)在乙醇 + 水混合物中的共溶剂效应。结果表明,乙醇质量分数为80%时,摩尔分数的最大溶解度为8.313×10 -2,大于纯水中的最大溶解度。溶剂效应分析表明,溶剂的氢键酸度(HBA)对尼马瑞韦的溶解度影响最大。富水区域的负优先溶剂化值表明 nirmatrelvir 分子优先被水分子溶剂化。随着共溶剂质量分数的增加,乙醇通过氢键破坏溶液中水分子的有序结构,使得尼马曲韦分子优先被乙醇溶剂化。尽管如此,在富含乙醇的区域,尼马曲韦分子优先再次被水溶剂化。通过分子动力学计算(MD)可以发现溶解行为与溶剂化自由能计算结果一致。此外,O·H2O(乙醇/水)和 NH·O(乙醇/水)的 RDF 图在 1.50–2.50 Å 处出现又高又尖的峰,表明乙醇和水分子形成规则的并通过氢键形式在 nirmatrelvir 部分周围以 1.50-2.50 Å 的距离形成明确的配位层。此外,通过Jouyban-Acree模型及其导数模型对溶解度数据进行相关拟合。此外,还完成了尼马瑞韦在乙醇水溶液中溶解过程的“表观热力学分析”,包括熵、焓和吉布斯自由能。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号