Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2023-09-02 , DOI: 10.1016/j.jwpe.2023.104208 Hao Li , Jiahao Zhang , Yiqi Du , Meixia Shi , Xinmou Kuang , Xiaolan Shen , Wenting Si
|
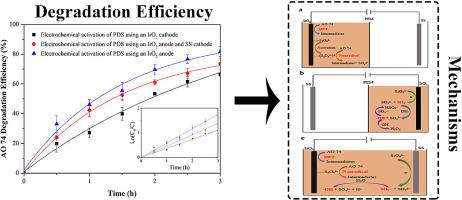
Electrochemical activation of persulfate is an efficient and green technology. In this study, the differences in the pollutant degradation efficiency and peroxydisulfate (PDS) activation mechanism were compared via electrochemical activation of PDS using only an IrO2 anode, an IrO2 cathode, and both an IrO2 anode and stainless steel (SS) cathode. In these methods, the degradation of acid orange (AO) 74 conformed to the pseudo-first-order kinetic degradation model. The electrochemical activation of PDS using an IrO2 anode resulted in the highest AO 74 degradation efficiency of 81.7 % with a degradation rate constant of 0.58 h−1. The results of radical quenching and cyclic voltammetry indicated that during the electrochemical activation of PDS using an IrO2 anode, direct electron transfer and nonradical oxidation contributed to AO 74 degradation. Using an IrO2 cathode, the contribution to AO 74 degradation was mainly  OH, while using an IrO2 anode and SS cathode, the contribution was the joint action of the nonradical and radical oxidation. Furthermore, during the electrochemical activation of PDS using an IrO2 anode, the degradation rate exhibited a good linear relationship with the current density and PDS concentration. The addition of Cl− considerably enhanced AO 74 degradation.
OH, while using an IrO2 anode and SS cathode, the contribution was the joint action of the nonradical and radical oxidation. Furthermore, during the electrochemical activation of PDS using an IrO2 anode, the degradation rate exhibited a good linear relationship with the current density and PDS concentration. The addition of Cl− considerably enhanced AO 74 degradation.
中文翻译:

使用IrO2电极电化学活化过二硫酸盐以有效降解酸性橙74:不同活化方法的机理
过硫酸盐的电化学活化是一种高效、绿色的技术。在本研究中,通过仅使用IrO 2阳极、IrO 2阴极以及IrO 2阳极和不锈钢(SS)阴极对PDS进行电化学活化,比较了污染物降解效率和过二硫酸盐(PDS)活化机制的差异。 。在这些方法中,酸性橙(AO)74的降解符合准一级动力学降解模型。使用IrO 2阳极对PDS进行电化学活化,AO 74 降解效率最高为81.7 %,降解速率常数为0.58 h -1。自由基猝灭和循环伏安法的结果表明,在使用IrO 2阳极电化学活化PDS的过程中,直接电子转移和非自由基氧化导致了AO 74 的降解。使用IrO 2阴极,AO 74 降解的贡献主要是 OH,而使用IrO 2阳极和SS阴极,贡献是非自由基和自由基氧化的共同作用。此外,在使用IrO 2阳极电化学活化PDS过程中,降解率与电流密度和PDS浓度呈现良好的线性关系。Cl -的添加显着增强了 AO 74 的降解。
OH,而使用IrO 2阳极和SS阴极,贡献是非自由基和自由基氧化的共同作用。此外,在使用IrO 2阳极电化学活化PDS过程中,降解率与电流密度和PDS浓度呈现良好的线性关系。Cl -的添加显着增强了 AO 74 的降解。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号