Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An efficient method to access spiro pseudoindoxyl ketones: evaluation of indoxyl and their N-benzylated derivatives for inhibition of the activity of monoamine oxidases
RSC Advances ( IF 3.9 ) Pub Date : 2023-08-22 , DOI: 10.1039/d3ra03641c Karuppaiah Perumal 1 , Jiseong Lee 2 , Sesuraj Babiola Annes 1 , Subburethinam Ramesh 1 , T M Rangarajan 3 , Bijo Mathew 4 , Hoon Kim 2
RSC Advances ( IF 3.9 ) Pub Date : 2023-08-22 , DOI: 10.1039/d3ra03641c Karuppaiah Perumal 1 , Jiseong Lee 2 , Sesuraj Babiola Annes 1 , Subburethinam Ramesh 1 , T M Rangarajan 3 , Bijo Mathew 4 , Hoon Kim 2
Affiliation
|
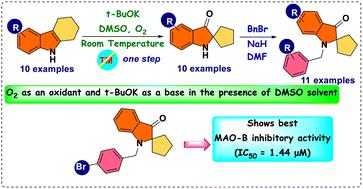
A simple, metal-free approach was developed to obtain novel pseudoindoxyl derivatives. The reaction was mediated by tBuOK on tetrahydrocarbazole 8 in dimethyl sulfoxide (DMSO) at room temperature through the hydroxylation of the indole double bond and a subsequent pinacol-type rearrangement. Spiro pseudoindoxyl compounds and their N-benzylated derivatives were assessed for their inhibitory activities against monoamine oxidase (MAO) enzymes. Based on half-maximal inhibitory concentration (IC50) values, 13 compounds were found to have higher inhibitory activity against MAO-B than against MAO-A. With regard to MAO-B inhibition, 11f showed the best inhibitory activity, with an IC50 value of 1.44 μM, followed by 11h (IC50 = 1.60 μM), 11j (IC50 = 2.78 μM), 11d (IC50 = 2.81 μM), and 11i (IC50 = 3.02 μM). Compound 11f was a competitive inhibitor with a Ki value of 0.51 ± 0.023 μM. In a reversibility experiment using dialysis, 11f showed effective recovery of MAO-B inhibition similar to that of safinamide. These experiments suggested that 11f was a potent, reversible, and competitive inhibitor of MAO-B activity.
中文翻译:

一种获取螺假吲哚酚酮的有效方法:评估吲哚酚及其 N-苄基化衍生物对单胺氧化酶活性的抑制作用
开发了一种简单的无金属方法来获得新型假吲哚酚衍生物。该反应由四氢咔唑8上的t BuOK 在二甲基亚砜 (DMSO) 中于室温下通过吲哚双键的羟基化和随后的频哪醇型重排介导。评估了螺假吲哚酚化合物及其N-苄基化衍生物对单胺氧化酶(MAO)的抑制活性。基于半最大抑制浓度(IC 50 )值,发现13种化合物对MAO-B的抑制活性高于对MAO-A的抑制活性。对于MAO-B抑制, 11f表现出最好的抑制活性,IC 50值为1.44 μM,其次是11h (IC 50 = 1.60 μM)、 11j (IC 50 = 2.78 μM)、 11d (IC 50 = 2.81)。 μM) 和11i (IC 50 = 3.02 μM)。化合物11f是一种竞争性抑制剂,K 值为 0.51 ± 0.023 μM。在使用透析的可逆性实验中, 11f显示出与 safinamide 类似的 MAO-B 抑制的有效恢复。这些实验表明11f是一种有效的、可逆的、竞争性的 MAO-B 活性抑制剂。
更新日期:2023-08-22
中文翻译:

一种获取螺假吲哚酚酮的有效方法:评估吲哚酚及其 N-苄基化衍生物对单胺氧化酶活性的抑制作用
开发了一种简单的无金属方法来获得新型假吲哚酚衍生物。该反应由四氢咔唑8上的t BuOK 在二甲基亚砜 (DMSO) 中于室温下通过吲哚双键的羟基化和随后的频哪醇型重排介导。评估了螺假吲哚酚化合物及其N-苄基化衍生物对单胺氧化酶(MAO)的抑制活性。基于半最大抑制浓度(IC 50 )值,发现13种化合物对MAO-B的抑制活性高于对MAO-A的抑制活性。对于MAO-B抑制, 11f表现出最好的抑制活性,IC 50值为1.44 μM,其次是11h (IC 50 = 1.60 μM)、 11j (IC 50 = 2.78 μM)、 11d (IC 50 = 2.81)。 μM) 和11i (IC 50 = 3.02 μM)。化合物11f是一种竞争性抑制剂,K 值为 0.51 ± 0.023 μM。在使用透析的可逆性实验中, 11f显示出与 safinamide 类似的 MAO-B 抑制的有效恢复。这些实验表明11f是一种有效的、可逆的、竞争性的 MAO-B 活性抑制剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号