当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroalkoxylation-Initiated Cascade on Sulfone-Tethered Aryl Alkynols Gives Cyclic and Spiro-Heterocyclic β-Ketosulfones
Organic Letters ( IF 4.9 ) Pub Date : 2023-08-10 , DOI: 10.1021/acs.orglett.3c02241 Santosh J Gharpure 1 , Dipak J Fartade 1 , Santosh K Nanda 1 , Shipra Somani 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-08-10 , DOI: 10.1021/acs.orglett.3c02241 Santosh J Gharpure 1 , Dipak J Fartade 1 , Santosh K Nanda 1 , Shipra Somani 1
Affiliation
|
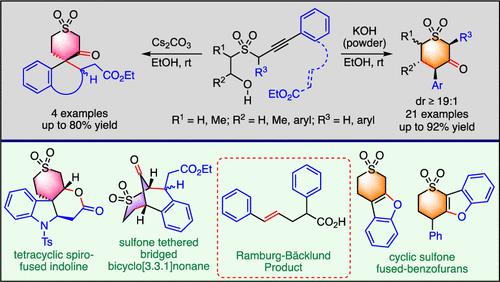
Serendipitous formation of cyclic β-ketosulfones is observed when sulfone-tethered arylalkynols are reacted with base. The reaction involves a base-promoted propargyl sulfone to the allene isomerization/intramolecular hydroalkoxylation/retro-oxa-Michael/6-endo-trig Michael addition cascade. Sulfone-tethered alkynyl acrylates gave stereoselective access to a diverse array of spirocyclic β-ketosulfone benzofuran/isochroman/indolines and sulfone-tethered bridged bicyclo[3.3.1]nonane. These cyclic β-ketosulfones could be readily elaborated into benzofuran-fused cyclic sulfones and tetracyclic spiroindoline.
中文翻译:

砜系芳基炔醇上的氢烷氧基化引发级联生成环状和螺杂环 β-酮砜
当砜系芳基炔醇与碱反应时,观察到环状 β-酮砜的偶然形成。该反应涉及碱促进的炔丙基砜至丙二烯异构化/分子内加氢烷氧基化/retro- oxa -Michael/6- end - trig Michael加成级联。砜系丙烯酸炔基酯可以立体选择性地获得多种螺环β-酮砜苯并呋喃/异色满/二氢吲哚和砜系桥联双环[3.3.1]壬烷。这些环状β-酮砜可以很容易地加工成苯并呋喃稠合的环状砜和四环螺吲哚啉。
更新日期:2023-08-10
中文翻译:

砜系芳基炔醇上的氢烷氧基化引发级联生成环状和螺杂环 β-酮砜
当砜系芳基炔醇与碱反应时,观察到环状 β-酮砜的偶然形成。该反应涉及碱促进的炔丙基砜至丙二烯异构化/分子内加氢烷氧基化/retro- oxa -Michael/6- end - trig Michael加成级联。砜系丙烯酸炔基酯可以立体选择性地获得多种螺环β-酮砜苯并呋喃/异色满/二氢吲哚和砜系桥联双环[3.3.1]壬烷。这些环状β-酮砜可以很容易地加工成苯并呋喃稠合的环状砜和四环螺吲哚啉。






























 京公网安备 11010802027423号
京公网安备 11010802027423号